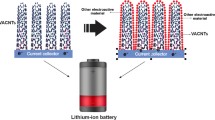
Abstract
Energy storage devices with high energy and power densities are highly attractive for various applications ranging from portable electronics to electric vehicles and grid-level energy storage, such as rechargeable batteries and supercapacitors. One limiting factor in power density is the ion transport in electrolyte, particularly in tortuous electrode materials with low porosity. A viable approach to enhance ion transport in electrolyte is to create vertically aligned structures and thus reduce electrode tortuosity. In the past decades, various methods have been explored to develop vertically aligned structures. This review summarizes battery kinetics to illustrate the importance of low tortuosity in electrodes, and then introduces various methods to create vertically aligned nanostructures, such as direct growth, templating and microfabrications. The electrochemical performance of electrodes or electrolytes created by each method is presented. At the end, this paper discusses challenges with these structures and the directions these technologies can be taken in the future.

Similar content being viewed by others
References
Larcher, D.; Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29.
Che, G. L.; Lakshmi, B. B.; Fisher, E. R.; Martin, C. R. Carbon nanotubule membranes for electrochemical energy storage and production. Nature 1998, 393, 346–349.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Augustyn, V.; Come, J.; Lowe, M. A.; Kim, J. W.; Taberna, P. L.; Tolbert, S. H.; Abruña, H. D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303.
Chabi, S.; Peng, C.; Hu, D.; Zhu, Y. Q. Ideal three-dimensional electrode structures for electrochemical energy storage. Adv. Mater. 2014, 26, 2440–2445.
Soloveichik, G. L. Battery technologies for large-scale stationary energy storage. Ann. Rev. Chem. Biomol. Eng. 2011, 2, 503–527.
Qian, G. Y.; Liao, X. B.; Zhu, Y. X.; Pan, F.; Chen, X.; Yang, Y. Designing flexible lithium-ion batteries by structural engineering. ACS Energy Lett. 2019, 4, 690–701.
Goodenough, J. B.; Park, K. S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
Guo, Y. G.; Hu, J. S.; Wan, L. J. Nanostructured materials for electrochemical energy conversion and storage devices. Adv. Mater. 2008, 20, 2878–2887.
Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group. Dusastre, V., Ed.; World Scientific: Hackensack, NJ, 2010; pp 160–170.
Thackeray, M. M.; Wolverton, C.; Isaacs, E. D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863.
Yao, P. C.; Zhu, B.; Zhai, H. W.; Liao, X. B.; Zhu, Y. X.; Xu, W. H.; Cheng, Q.; Jayyosi, C.; Li, Z.; Zhu, J. et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Lett. 2018, 18, 6113–6120.
Cheng, Q.; Xu, W. H.; Qin, S. Y.; Das, S.; Jin, T. W.; Li, A. J.; Li, A. C.; Qie, B. Y.; Yao, P. C.; Zhai, H. W. et al. Full dissolution of the whole lithium sulfide family (Li2S8 to Li2S) in a safe eutectic solvent for rechargeable lithium-sulfur batteries. Angew. Chem., Int. Ed. 2019. (in press)
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group. Dusastre, V., Ed.; World Scientific: Hackensack, NJ, 2010; pp 171–179.
Scrosati, B.; Hassoun, J.; Sun, Y. K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295.
Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group. Dusastre, V., Ed.; World Scientific: Hackensack, NJ, 2010; pp 320–329.
Lin, D. C.; Liu, W.; Liu, Y. Y.; Lee, H. R.; Hsu, P. C.; Liu, K.; Cui, Y. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett. 2015, 16, 459–465.
Fergus, J. W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569.
Shi, Y.; Zhou, X. Y.; Yu, G. H. Material and structural design of novel binder systems for high-energy, high-power lithium-ion batteries. Acc. Chem. Res. 2017, 50, 2642–2652.
Li, H. S.; Peng, L. L.; Zhu, Y.; Zhang, X. G.; Yu, G. H. Achieving high-energy-high-power density in a flexible quasi-solid-state sodium ion capacitor. Nano Lett. 2016, 16, 5938–5943.
Zhao, F.; Shi, Y.; Pan, L. J.; Yu, G. H. Multifunctional nanostructured conductive polymer gels: Synthesis, properties, and applications. Acc. Chem. Res. 2017, 50, 1734–1743.
Shi, Y.; Zhang, J.; Bruck, A. M.; Zhang, Y. M.; Li, J.; Stach, E. A.; Takeuchi, K. J.; Marschilok, A. C.; Takeuchi, E. S.; Yu, G. H. A tunable 3D nanostructured conductive gel framework electrode for high-performance lithium ion batteries. Adv. Mater. 2017, 29, 1603922.
Mirvakili, S. M.; Hunter, I. W. Vertically aligned niobium nanowire arrays for fast-charging micro-supercapacitors. Adv. Mater. 2017, 29, 1700671.
Melechko, A. V.; Merkulov, V. I.; McKnight, T. E.; Guillorn, M. A.; Klein, K. L.; Lowndes, D. H.; Simpson, M. L. Vertically aligned carbon nanofibers and related structures: Controlled synthesis and directed assembly. J. Appl. Phys. 2005, 97, 041301.
Mai, L. Q.; Tian, X. C.; Xu, X.; Chang, L.; Xu, L. Nanowire electrodes for electrochemical energy storage devices. Chem. Rev. 2014, 114, 11828–11862.
Li, X. J.; Wu, Y. C.; Hua, K.; Li, S.; Fang, D.; Luo, Z. P.; Bao, R.; Fan, X.; Yi, J. H. Vertically aligned polyaniline nanowire arrays for lithium-ion battery. Colloid Polym. Sci. 2018, 296, 1395–1400.
In, J. B.; Grigoropoulos, C. P.; Chernov, A. A.; Noy, A. Growth kinetics of vertically aligned carbon nanotube arrays in clean oxygen-free conditions. ACS Nano 2011, 5, 9602–9610.
Honda, Y.; Haramoto, T.; Takeshige, M.; Shiozaki, H.; Kitamura, T.; Ishikawa, M. Aligned MWCNT sheet electrodes prepared by transfer methodology providing high-power capacitor performance. Electrochem. Solid-State Lett. 2007, 10, A106–A110.
Sander, J. S.; Erb, R. M.; Li, L.; Gurijala, A.; Chiang, Y. M. High-performance battery electrodes via magnetic templating. Nat. Energy 2016, 1, 16099.
Billaud, J.; Bouville, F.; Magrini, T.; Villevieille, C.; Studart, A. R. Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries. Nat. Energy 2016, 1, 16097.
Li, L. S.; Erb, R. M.; Wang, J. J.; Wang, J.; Chiang, Y. M. Fabrication of low-tortuosity ultrahigh-area-capacity battery electrodes through magnetic alignment of emulsion-based slurries. Adv. Energy Mater. 2019, 9, 1802472.
Behr, S.; Amin, R.; Chiang, Y. M.; Tomsia, A. P. Highly-structured, additive-free lithium-ion cathodes by freeze-casting technology. Ceram. Forum Int. 2015, 92, 39–43.
Roberts, A. D.; Li, X.; Zhang, H. F. Hierarchically porous sulfur-containing activated carbon monoliths via ice-templating and one-step pyrolysis. Carbon 2015, 95, 268–278.
Zhai, H. W.; Xu, P. Y.; Ning, M. Q.; Cheng, Q.; Mandal, J.; Yang, Y. A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett. 2017, 17, 3182–3187.
Zhu, M. W.; Song, J. W.; Li, T.; Gong, A.; Wang, Y. B.; Dai, J. Q.; Yao, Y. G.; Luo, W.; Henderson, D.; Hu, L. B. Highly anisotropic, highly transparent wood composites. Adv. Mater. 2016, 28, 5181–5187.
Lu, L. L.; Lu, Y. Y.; Xiao, Z. J.; Zhang, T. W.; Zhou, F.; Ma, T.; Ni, Y.; Yao, H. B.; Yu, S. H.; Cui, Y. Wood-inspired high-performance ultrathick bulk battery electrodes. Adv. Mater. 2018, 30, 1706745.
Li, T.; Zhu, M. W.; Yang, Z.; Song, J. W.; Dai, J. Q.; Yao, Y. G.; Luo, W.; Pastel, G.; Yang, B.; Hu, L. B. Wood composite as an energy efficient building material: Guided sunlight transmittance and effective thermal insulation. Adv. Energy Mater. 2016, 6, 1601122.
Liu, T.; Wang, Y.; Zhang, Y. X.; Fang, S. M.; Lei, L. B.; Ren, C.; Chen, F. L. Steam electrolysis in a solid oxide electrolysis cell fabricated by the phase-inversion tape casting method. Electrochem. Commun. 2015, 61, 106–109.
Wang, C. L.; Taherabadi, L.; Jia, G. Y.; Madou, M.; Yeh, Y.; Dunn, B. C-MEMS for the manufacture of 3D microbatteries. Electrochem. Solid-State Lett. 2004, 7, A435–A438.
Chen, C. J.; Zhang, Y.; Li, Y. J.; Kuang, Y. D.; Song, J. W.; Luo, W.; Wang, Y. B.; Yao, Y. G.; Pastel, G.; Xie, J. et al. Highly conductive, lightweight, low-tortuosity carbon frameworks as ultrathick 3D current collectors. Adv. Energy Mater. 2017, 7, 1700595.
Jin, S.; Jiang, Y.; Ji, H. X.; Yu, Y. Advanced 3D current collectors for lithium-based batteries. Adv. Mater. 2018, 30, 1802014.
Newman, J.; Thomas-Alyea, K. E. Electrochemical Systems; 3rd ed. John Wiley & Sons: Hoboken, N.J., 2004.
Song, H. W.; Li, N.; Cui, H.; Wang, C. X. Enhanced storage capability and kinetic processes by pores- and hetero-atoms-riched carbon nanobubbles for lithium-ion and sodium-ion batteries anodes. Nano Energy 2014, 4, 81–87.
Fergus, J. W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954.
Tliha, M.; Khaldi, C.; Boussami, S.; Fenineche, N.; El-Kedim, O.; Mathlouthi, H.; Lamloumi, J. Kinetic and thermodynamic studies of hydrogen storage alloys as negative electrode materials for Ni/MH batteries: A review. J. Solid State Electroche. 2014, 18, 577–593.
Feng, F.; Geng, M.; Northwood, D. O. Electrochemical behaviour of intermetallic-based metal hydrides used in Ni/metal hydride (MH) batteries: A review. Int. J. Hydrogen Energy 2001, 26, 725–734.
Chen, J. H.; Li, W. Z.; Wang, D. Z.; Yang, S. X.; Wen, J. G.; Ren, Z. F. Electrochemical characterization of carbon nanotubes as electrode in electrochemical double-layer capacitors. Carbon 2002, 40, 1193–1197.
Bohlen, O.; Kowal, J.; Sauer, D. U. Ageing behaviour of electrochemical double layer capacitors: Part I. Experimental study and ageing model. J. Power Sources 2007, 172, 468–475.
Thorat, I. V.; Stephenson, D. E.; Zacharias, N. A.; Zaghib, K.; Harb, J. N.; Wheeler, D. R. Quantifying tortuosity in porous Li-ion battery materials. J. Power Sources 2009, 188, 592–600.
Ebner, M.; Chung, D. W.; García, R. E.; Wood, V. Tortuosity anisotropy in lithium-ion battery electrodes. Adv. Energy Mater. 2014, 4, 1301278.
Pouraghajan, F.; Knight, H.; Wray, M.; Mazzeo, B.; Subbaraman, R.; Christensen, J.; Wheeler, D. Quantifying tortuosity of porous Li-ion battery electrodes: Comparing polarization-interrupt and blocking-electrolyte methods. J. Electrochem. Soc. 2018, 165, A2644–A2653.
DuBeshter, T.; Sinha, P. K.; Sakars, A.; Fly, G. W.; Jorne, J. Measurement of tortuosity and porosity of porous battery electrodes. J. Electrochem. Soc. 2014, 161, A599–A605.
Suthar, B.; Landesfeind, J.; Eldiven, A.; Gasteiger, H. A. Method to determine the in-plane tortuosity of porous electrodes. J. Electrochem. Soc. 2018, 165, A2008–A2018.
Forouzan, M. M.; Wray, M.; Robertson, L.; Wheeler, D. R. Tortuosity of composite porous electrodes with various conductive additives in an alkaline system. J. Electrochem. Soc. 2017, 164, A3117–A3130.
Shen, L. F.; Yuan, C. Z.; Luo, H. J.; Zhang, X. G.; Xu, K.; Xia, Y. Y. Facile synthesis of hierarchically porous Li4Ti5O12 microspheres for high rate lithium ion batteries. J. Mater. Chem. 2010, 20, 6998–7004.
Mirvakili, S. M.; Pazukha, A.; Sikkema, W.; Sinclair, C. W.; Spinks, G. M.; Baughman, R. H.; Madden, J. D. W. Niobium nanowire yarns and their application as artificial muscles. Adv. Funct. Mater. 2013, 23, 4311–4316.
Zhu, J. X.; Sakaushi, K.; Clavel, G.; Shalom, M.; Antonietti, M.; Fellinger, T. P. A general salt-templating method to fabricate vertically aligned graphitic carbon nanosheets and their metal carbide hybrids for superior lithium ion batteries and water splitting. J. Am. Chem. Soc. 2015, 137, 5480–5485.
Hsia, B.; Marschewski, J.; Wang, S.; In, J. B.; Carraro, C.; Poulikakos, D.; Grigoropoulos, C. P.; Maboudian, R. Highly flexible, all solid-state micro-supercapacitors from vertically aligned carbon nanotubes. Nanotechnology 2014, 25, 055401.
Bae, J.; Song, M. K.; Park, Y. J.; Kim, J. M.; Liu, M. L.; Wang, Z. L. Fiber supercapacitors made of nanowire-fiber hybrid structures for wearable/flexible energy storage. Angew. Chem., Int. Ed. 2011, 50, 1683–1687.
Peng, S. J.; Li, L. L.; Wu, H. B.; Madhavi, S.; Lou, X. W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172.
Shen, L. F.; Uchaker, E.; Zhang, X. G.; Cao, G. Z. Hydrogenated Li4Ti5O12 nanowire arrays for high rate lithium ion batteries. Adv. Mater. 2012, 24, 6502–6506.
Xu, J. S.; Zhu, Y. J. Monodisperse Fe3O4 and γ-Fe2O3 magnetic mesoporous microspheres as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4752–4757.
Zhang, G. H.; Hou, S. C.; Zhang, H.; Zeng, W.; Yan, F. L.; Li, C. C.; Duan, H. G. High-performance and ultra-stable lithium-ion batteries based on MOF-derived ZnO@ZnO quantum dots/C core-shell nanorod arrays on a carbon cloth anode. Adv. Mater. 2015, 27, 2400–2405.
Yuan, K.; Guo-Wang, P.; Hu, T.; Shi, L.; Zeng, R.; Forster, M.; Pichler, T.; Chen, Y. W.; Scherf, U. Nanofibrous and graphene-templated conjugated microporous polymer materials for flexible chemosensors and supercapacitors. Chem. Mater. 2015, 27, 7403–7411.
Wang, Z. L.; Guo, R.; Ding, L. X.; Tong, Y. X.; Li, G. R. Controllable template-assisted electrodeposition of single-and multi-walled nanotube arrays for electrochemical energy storage. Sci. Rep. 2013, 3, 1204.
Roberts, A. D.; Li, X.; Zhang, H. F. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356.
Cheng, J. L.; Gu, G. F.; Guan, Q.; Razal, J. M.; Wang, Z. Y.; Li, X. L.; Wang, B. Synthesis of a porous sheet-like V2O5-CNT nanocomposite using an ice-templating “bricks-and-mortar” assembly approach as a high-capacity, long cyclelife cathode material for lithium-ion batteries. J. Mater. Chem. A. 2016, 4, 2729–2737.
Wang, Y.; Kong, D. Z.; Shi, W. H.; Liu, B.; Sim, G. J.; Ge, Q.; Yang, H. Y. Ice templated free-standing hierarchically WS2/CNT-rGO aerogel for high-performance rechargeable lithium and sodium ion batteries. Adv. Energy Mater. 2016, 6, 1601057.
Roberts, A. D.; Wang, S. X.; Li, X.; Zhang, H. F. Hierarchical porous nitrogen-rich carbon monoliths via ice-templating: High capacity and high-rate performance as lithium-ion battery anode materials. J. Mater. Chem. A 2014, 2, 17787–17796.
Cheng, J. L.; Gu, G. F.; Guan, Q.; Razal, J. M.; Wang, Z. Y.; Li, X. L.; Wang, B. Synthesis of a porous sheet-like V2O5-CNT nanocomposite using an ice-templating “bricks-and-mortar” assembly approach as a high-capacity, long cyclelife cathode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 2729–2737.
Needham, S. A.; Wang, G. X.; Liu, H. K. Synthesis of NiO nanotubes for use as negative electrodes in lithium ion batteries. J. Power Sources 2006, 159, 254–257.
Wang, K.; Wu, H. P.; Meng, Y. N.; Wei, Z. X. Conducting polymer nanowire arrays for high performance supercapacitors. Small 2014, 10, 14–31.
Taberna, P. L.; Mitra, S.; Poizot, P.; Simon, P.; Tarascon, J. M. High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications. Nat. Mater. 2006, 5, 567–573.
Lee, S.; Cho, M. S.; Nam, J. D.; Lee, Y. Fabrication of polypyrrole nanorod arrays for supercapacitor: Effect of length of nanorods on capacitance. J. Nanosci. Nanotechnol. 2008, 8, 5036–5041.
Zhou, Y. K.; Shen, C. M.; Li, H. L. Synthesis of high-ordered LiCoO2 nanowire arrays by AAO template. Solid State Ionics 2002, 146, 81–86.
Cheng, F. Y.; Tao, Z. L.; Liang, J.; Chen, J. Template-directed materials for rechargeable lithium-ion batteries. Chem. Mater. 2008, 20, 667–681.
Shi, N.; Su, F.; Huan, D. M.; Xie, Y.; Lin, J.; Tan, W. Z.; Peng, R. R.; Xia, C. R.; Chen, C. S.; Lu, Y. L. Performance and DRT analysis of P-SOFCs fabricated using new phase inversion combined tape casting technology. J. Mater. Chem. A 2017, 5, 19664–19671.
Pearse, A.; Schmitt, T.; Sahadeo, E.; Stewart, D. M.; Kozen, A.; Gerasopoulos, K.; Talin, A. A.; Lee, S. B.; Rubloff, G. W.; Gregorczyk, K. E. Three-dimensional solid-state lithium-ion batteries fabricated by conformal vapor-phase chemistry. ACS Nano 2018, 12, 4286–4294.
Su, D. S.; Schlögl, R. Nanostructured carbon and carbon nanocomposites for electrochemical energy storage applications. ChemSusChem 2010, 3, 136–168.
Li, J.; Zhang, G. F.; Chen, N.; Nie, X. W.; Ji, B. X.; Qu, L. T. Built structure of ordered vertically aligned codoped carbon nanowire arrays for supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 24840–24845.
Fan, Y.; Zhang, Q.; Xiao, Q. Z.; Wang, X. H.; Huang, K. High performance lithium ion battery anodes based on carbon nanotube-silicon core-shell nanowires with controlled morphology. Carbon 2013, 59, 264–269.
Chen, K. F.; Song, S. Y.; Liu, F.; Xue, D. F. Structural design of graphene for use in electrochemical energy storage devices. Chem. Soc. Rev. 2015, 44, 6230–6257.
Basiricò, L.; Lanzara, G. Moving towards high-power, high-frequency and low-resistance CNT supercapacitors by tuning the CNT length, axial deformation and contact resistance. Nanotechnology 2012, 23, 305401.
Amade, R.; Jover, E.; Caglar, B.; Mutlu, T.; Bertran, E. Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application. J. Power Sources 2011, 196, 5779–5783.
Portet, C.; Yushin, G.; Gogotsi, Y. Electrochemical performance of carbon onions, nanodiamonds, carbon black and multiwalled nanotubes in electrical double layer capacitors. Carbon 2007, 45, 2511–2518.
Yoon, B. J.; Jeong, S. H.; Lee, K. H.; Kim, H. S.; Park, C. G.; Han, J. H. Electrical properties of electrical double layer capacitors with integrated carbon nanotube electrodes. Chem. Phys. Lett. 2004, 388, 170–174.
Xu, Y.; Zhou, M.; Lei, Y. Nanoarchitectured array electrodes for rechargeable lithium- and sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1502514.
Peng, S. J.; Li, L. L.; Wu, H. B.; Madhavi, S.; Lou, X. W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172.
Vayssieres, L.; Graetzel, M. Highly ordered SnO2 nanorod arrays from controlled aqueous growth. Angew. Chem., Int. Ed. 2004, 43, 3666–3670.
Ma, R.; Bando, Y.; Zhang, L.; Sasaki, T. Layered MnO2 nanobelts: Hydrothermal synthesis and electrochemical measurements. Adv. Mater. 2004, 16, 918–922.
Wang, Y.; Kong, D. Z.; Shi, W. H.; Liu, B.; Sim, G. J.; Ge, Q.; Yang, H. Y. Ice templated free-standing hierarchically WS2/CNT-rGO aerogel for high-performance rechargeable lithium and sodium ion batteries. Adv. Energy Mater. 2016, 6, 1601057.
Gutiérrez, M. C.; Jobbágy, M.; Rapún, N.; Ferrer, M. L.; Monte, F. A biocompatible bottom-up route for the preparation of hierarchical biohybrid materials. Adv. Mater. 2006, 18, 1137–1140.
Li, Y.; Wu, C.; Bai, Y.; Liu, L.; Wang, H.; Wu, F.; Zhang, N.; Zou, Y. F. Hierarchical mesoporous lithium-rich Li[Li0.2Ni0.2Mn0.6]O2 cathode material synthesized via ice templating for lithium-ion battery. ACS Appl. Mater. Interfaces 2016, 8, 18832–18840.
Huang, C.; Grant, P. S. Coral-like directional porosity lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2018, 6, 14689–14699.
Huang, Y. S.; Wu, D. Q.; Jiang, J. Z.; Mai, Y. Y.; Zhang, F.; Pan, H.; Feng, X. L. Highly oriented macroporous graphene hybrid monoliths for lithium ion battery electrodes with ultrahigh capacity and rate capability. Nano Energy 2015, 12, 287–295.
Wu, H.; Chan, G.; Choi, J. W.; Ryu, I.; Yao, Y.; McDowell, M. T.; Lee, S. W.; Jackson, A.; Yang, Y.; Hu, L. B. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315.
Kim, J. G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M. J.; Chung, H. Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322.
Suo, L. M.; Hu, Y. S.; Li, H.; Armand, M.; Chen, L. Q. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481.
Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A. et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392.
Zhang, X. K.; Xie, J.; Shi, F. F.; Lin, D. C.; Liu, Y. Y.; Liu, W.; Pei, A.; Gong, Y. J.; Wang, H. X.; Liu, K. et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett. 2018, 18, 3829–3838.
Usseglio-Viretta, F. L. E.; Colclasure, A.; Mistry, A. N.; Claver, K. P. Y.; Pouraghajan, F.; Finegan, D. P.; Heenan, T. M. M.; Abraham, D.; Mukherjee, P. P.; Wheeler, D. et al. Resolving the discrepancy in tortuosity factor estimation for li-ion battery electrodes through micro-macro modeling and experiment. J. Electrochem. Soc. 2018, 165, A3403–A3426.
Zhao, Y.; Liu, B. R.; Pan, L. J.; Yu, G. H. 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ. Sci. 2013, 6, 2856–2870.
Xiao, R.; Cho, S. I.; Liu, R.; Lee, S. B. Controlled electrochemical synthesis of conductive polymer nanotube structures. J. Am. Chem. Soc. 2007, 129, 4483–4489.
Wang, Z. L.; Guo, R.; Ding, L. X.; Tong, Y. X.; Li, G. R. Controllable template-assisted electrodeposition of single- and multi-walled nanotube arrays for electrochemical energy storage. Sci. Rep. 2013, 3, 1204.
Wang, Y.; Fu, X. W.; Zheng, M.; Zhong, W. H.; Cao, G. Z. Strategies for building robust traffic networks in advanced energy storage devices: A focus on composite electrodes. Adv. Mater. 2019, 31, 1804204.
Ruiz-Morales, J. C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Marín Rueda, J. R.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859.
Long, J. W.; Dunn, B.; Rolison, D. R.; White, H. S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492.
Arthur, T. S.; Bates, D. J.; Cirigliano, N.; Johnson, D. C.; Malati, P.; Mosby, J. M.; Perre, E.; Rawls, M. T.; Prieto, A. L.; Dunn, B. Three-dimensional electrodes and battery architectures. MRS Bull. 2011, 36, 523–531.
Liu, J.; Zhu, H. Z.; Shiraz, M. H. A. Toward 3D solid-state batteries via atomic layer deposition approach. Front. Energy Res. 2018, 6, 10.
Cirigliano, N.; Sun, G.Y.; Membreno, D.; Malati, P.; Kim, C. J.; Dunn, B. 3D architectured anodes for lithium-ion microbatteries with large areal capacity. Energy Technol. 2014, 2, 362–369.
Baggetto, L.; Niessen, R. A. H.; Roozeboom, F.; Notten, P. H. L. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Functional Mater. 2008, 18, 7, 1057–1066.
Hur, J. I.; Smith, L. C.; Dunn, B. High areal energy density 3D lithium-ion microbatteries. Joule 2018, 2, 1187–1201.
Deville, S. Freezing Colloids: Observations, Principles, Control, and Use: Applications in Materials Science, Life Science, Earth Science, Food Science, and Engineering; Springer: Cham, 2017.
Acknowledgements
Yuan Yang acknowledges the funding support from AFOSR (No. FA9550-18-1-0410). Xue Wang thank the financial support from China Scholarship Council during her study (CSC, No. 201706120088).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, X., Wang, T., Borovilas, J. et al. Vertically-aligned nanostructures for electrochemical energy storage. Nano Res. 12, 2002–2017 (2019). https://doi.org/10.1007/s12274-019-2392-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2392-x