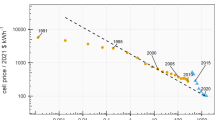
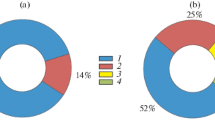
Abstract
Increasing energy consumption, shortages of fossil fuels, and concerns about the environmental impact of energy use, especially emissions of carbon dioxide, give fresh impetus to the development of renewable energy sources. With the advent of renewable energy, it is now indispensable that efficient energy storage systems have to be developed. One of the most promising storage systems to be employed in stationary energy storage applications are lithium-based batteries (LIB), mainly due to their high energy density, high power, and nearly 100 % efficiency. Within the scope of this paper, we carry out a patent search using the patent database PatBase® to assess the development status of LIB technology. The analysis of the generated patent sample reveals disproportionately high growth rates in LIB patent applications over the last years compared to other selected energy-related technologies. Breaking down patent application growth by the different components of LIB shows the principal drivers of growth. The purpose of this paper is to provide current research trends and prospects for the main LIB materials and designs.










Similar content being viewed by others
Notes
For further Information on LIB see: [9].
For details see: http://www.epo.org/searching/essentials/patent-families.html, accessed November 2012.
These patent family applications are subsequently termed as patent families, whereby each patent family consists of at least one patent application without regard to the status of being granted later on.
Source: PatBase®, July 2012.
For details see: [68].
References
EEA (2008) Energy and environment report 2008. European Environment Agency (EEA), Copenhagen
BMU (2011) Renewable Energy Sources in Figures. Federal ministry for the Environment, nature Conservation and nuclear Safety (BMU), Berlin
Winter M, Brodd RJ (2004) What are batteries, fuel cells, and supercapacitors? Chem Rev 104(10):4245–4270. doi:10.1021/cr020730k
Hall PJ, Bain EJ (2008) Energy-storage technologies and electricity generation. Energy Policy 36(12):4352–4355
Winter M, Passerini S (2011) Lithium ion batteries as key component for energy storage in automotive and stationary applications. In: Telecommunications energy conference (INTELEC), 2011 IEEE 33rd International, 9–13 Oct, pp 1–3. doi:10.1109/intlec.2011.6099839
Winter M, Besenhard JO (1999) Wiederaufladbare Batterien. Chem unserer Zeit 33(6):320–332. doi:10.1002/ciuz.19990330603
Beaudin M, Zareipour H, Schellenberglabe A, Rosehart W (2010) Energy storage for mitigating the variability of renewable electricity sources: an updated review. Energy Sustain Dev 14(4):302–314
Besenhard JO, Winter M (1998) Insertion reactions in advanced electrochemical energy storage. Pure Appl Chem 70:603–608. doi:10.1351/pac199870030603
Linden D, Reddy TB (2002) Handbook of batteries, 3rd edn. McGraw-Hill, New York
Debackere K, Verbeek A, Luwel M, Zimmermann E (2002) Measuring progress and evolution in science and technology—II: the multiple uses of technometric indicators. Int J Manag Rev 4(3):213–231
Bregonje M (2005) Patents: a unique source for scientific technical information in chemistry related industry? World Patent Inf 27(4):309–315
Ernst H (1997) The use of patent data for technological forecasting: the diffusion of CNC-technology in the machine tool industry. Small Bus Econ 9(4):361–381. doi:10.1023/a:1007921808138
Merino DN (1990) Development of a technological S-curve for tire cord textiles. Technol Forecast Soc Chang 37(3):275–291
Ernst H (1995) Patenting strategies in the German mechanical engineering industry and their relationship to company performance. Technovation 15(4):225–240
Griliches Z, Hall BH, Pakes A (1991) R&D, patents, and market value revisited: is there a second (technological opportunity) factor? Econ Innov New Technol 1(3):183–201. doi:10.1080/10438599100000001
Schwander P (2000) An evaluation of patent searching resources: comparing the professional and free on-line databases. World Patent Inf 22(3):147–165
Tseng Y-H, Lin C-J, Lin Y-I (2007) Text mining techniques for patent analysis. Inf Process Manag 43(5):1216–1247
Yoshio M, Brodd RJ, Kozawa A (2009) Lithium-ion batteries: science and technologies. Springer, New York
Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D (2011) A review of advanced and practical lithium battery materials. J Mater Chem 21(27):9938–9954. doi:10.1039/c0jm04225k
The High-power Lithium-ion (2012) Cadex Electronics Inc. (Cadex)—Battery University. http://batteryuniversity.com/learn/article/the_high_power_lithium_ion/. Accessed 8 Nov 2012
Goodenough JB, Mizuchima K (1980) Electrochemical cell with new fast ion conductors. United States Patent US4302518, 1981/11/24
Ohzuku T, Brodd RJ (2007) An overview of positive-electrode materials for advanced lithium-ion batteries. J Power Sources 174(2):449–456
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104(10):4271–4301. doi:10.1021/Cr020731c
Xu B, Qian D, Wang Z, Meng YS (2012) Recent progress in cathode materials research for advanced lithium ion batteries. Mater Sci Eng 73(5–6):51–65
Yamaki J (2009) Secondary batteries—lithium rechargeable systems—lithium-ion. Overview. Elsevier, Amsterdam
Belharouak I, Sun YK, Liu J, Amine K (2003) Li(Ni1/3Co1/3Mn1/3)O2 as a suitable cathode for high power applications. J Power Sources 123(2):247–252
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4(9):3243–3262
Kim J, Hong Y, Ryu KS, Kim MG, Cho J (2006) Washing effect of a LiNi0.83Co0.15Al0.02O2 cathode in water. Electrochem Solid-State Lett 9:A19–A23. doi:10.1149/1.2135427
Zhuang GV, Chen G, Shim J, Song X, Ross PN, Richardson TJ (2004) Li2CO3 in LiNi0.8Co0.15Al0.05O2 cathodes and its effects on capacity and power. J Power Sources 134(2):293–297. doi:10.1016/j.jpowsour.2004.02.030
Ohzuku T, Makimura Y (2001) Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem Lett 30(7):642–643
Li J, Klöpsch R, Stan MC, Nowak S, Kunze M, Winter M, Passerini S (2011) Synthesis and electrochemical performance of the high voltage cathode material Li[Li0.2Mn0.56Ni0.16Co0.08]O2 with improved rate capability. J Power Sources 196(10):4821–4825. doi:10.1016/j.jpowsour.2011.01.006
Lu Z, Dahn JR (2001) Cathode Compositions for Lithium-ion batteries. United States Patent US6964828, 2005/15/11
Thackeray MM, Johnson CS, Khalil A, Jaekook K (2001) Lithium metal oxide electrodes for lithium cells and batteries. United States Patent US20020136954, 2002/26/09
Thackeray MM, Kang S-H, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17(30):3112–3125
Li J, Klöpsch R, Nowak S, Kunze M, Winter M, Passerini S (2011) Investigations on cellulose-based high voltage composite cathodes for lithium ion batteries. J Power Sources 196(18):7687–7691. doi:10.1016/j.jpowsour.2011.04.030
Amatucci G, Tarascon J-M (2002) Optimization of insertion compounds such as LiMn2O4 for Li-ion batteries. J Electrochem Soc 149(12):K31–K46. doi:10.1149/1.1516778
Thackeray MM, Goodenough JB (1982) Solid state cell wherein an anode, solid electrolyte and cathode each comprise a cubic-close-packed framework structure. United States Patent US4507371, 1985/26/03
Zhong Q, Bonakdarpour A (1996) High voltage insertion compounds for lithium batteries. United States Patent US5631104, 1997/05/20
Goodenough JB, Padhi AK, Nanjundaswamy KS, Masquelier C (1997) Cathode materials for secondary (rechargeable) lithium batteries. United States Patent US5910382, 1999/06/08
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144(4):1188–1194. doi:10.1149/1.1837571
Padhi AK, Nanjundaswamy KS, Masquelier C, Okada S, Goodenough JB (1997) Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J Electrochem Soc 144(5):1609–1613. doi:10.1149/1.1837649
Winter M, Besenhard JO, Spahr ME, Novák P (1998) Insertion electrode materials for rechargeable lithium batteries. Adv Mater 10(10):725–763. doi:10.1002/(sici)1521-4095(199807)10:10<725:aid-adma725>3.0.co;2-z
Besenhard JO, Winter M (2002) Advances in battery technology: rechargeable magnesium batteries and novel negative-electrode materials for lithium ion batteries. ChemPhysChem 3:155–159. doi:10.1002/1439-7641(20020215)3:2<155:aid-cphc155>3.0.co;2-s
Hayner CM, Zhao X, Kung HH (2012) Materials for rechargeable lithium-ion batteries. Annu Rev Chem Biomol Eng 3(1):445–471. doi:10.1146/annurev-chembioeng-062011-081024
Placke T, Bieker P, Lux SF, Fromm O, Meyer H-W, Passerini S, Winter M (2012) Dual-ion cells based on anion intercalation into graphite from ionic liquid-based electrolytes. Z Phys Chem 226(5–6):391–407. doi:10.1524/zpch.2012.0222
Placke T, Fromm O, Lux SF, Bieker P, Rothermel S, Meyer H-W, Passerini S, Winter M (2012) Reversible intercalation of bis(trifluoromethanesulfonyl)imide anions from an ionic liquid electrolyte into graphite for high performance dual-ion cells. J Electrochem Soc 159(11):A1755–A1765. doi:10.1149/2.011211jes
Placke T, Siozios V, Schmitz R, Lux SF, Bieker P, Colle C, Meyer HW, Passerini S, Winter M (2012) Influence of graphite surface modifications on the ratio of basal plane to “non-basal plane” surface area and on the anode performance in lithium ion batteries. J Power Sources 200:83–91
Novák P, Scheifele W, Winter M, Haas O (1997) Graphite electrodes with tailored porosity for rechargeable ion-transfer batteries. J Power Sources 68(2):267–270
Zheng T, Xue JS, Dahn JR (1996) Lithium insertion in hydrogen-containing carbonaceous materials. Chem Mater 8(2):389–393. doi:10.1021/cm950304y
Kaskhedikar NA, Maier J (2009) Lithium storage in carbon nanostructures. Adv Mater 21:2664–2680. doi:10.1002/adma.200901079
Dey AN (1971) Electrochemical alloying of lithium in organic electrolytes. J Electrochem Soc 118(10):1547–1549. doi:10.1149/1.2407783
Yoshio M, Wang H, Fukuda K, Umeno T, Dimov N, Ogumi Z (2002) Carbon-coated Si as a lithium-ion battery anode material. J Electrochem Soc 149:A1598–A1603. doi:10.1149/1.1518988
Liu W-R, Yen Y-C, Wu H-C, Winter M, Wu N-L (2009) Nano-porous SiO/carbon composite anode for lithium-ion batteries. J Appl Electrochem 39:1643–1649. doi:10.1007/s10800-009-9854-x
Park C-M, Kim J-H, Kim H, Sohn H-J (2010) Li-alloy based anode materials for Li secondary batteries. Chem Soc Rev 39(8):3115–3141
Idota Y (1993) Nonaqueous secondary battery. United States Patent US5478671, 1995/12/26
Yang J, Takeda Y, Imanishi N, Capiglia C, Xie JY, Yamamoto O (2002) SiOx-based anodes for secondary lithium batteries. Solid State Ionics 152–153:125–129
Winter M, Besenhard JO, Albering JH, Yang J, Wachtler M (1998) Lithium storage alloys as anode materials in lithium ion batteries. Prog Batter Battery Mater 17:208–213
Winter M, Appel WK, Evers B, Hodal T, Möller K-C, Schneider I, Wachtler M, Wagner MR, Wrodnigg GH, Besenhard JO (2001) Studies on the anode/electrolyte interfacein lithium ion batteries. Monatsh Chem 132(4):473–486. doi:10.1007/s007060170110
Winter M (2009) The solid electrolyte interphase—the most important and the least understood solid electrolyte in rechargeable Li batteries. Z Phys Chem 223(10–11):1395–1406. doi:10.1524/zpch.2009.6086
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4417. doi:10.1021/Cr030203g
Aurbach D, Talyosef Y, Markovsky B, Markevich E, Zinigrad E, Asraf L, Gnanaraj JS, Kim H-J (2004) Design of electrolyte solutions for Li and Li-ion batteries: a review. Electrochim Acta 50(2–3):247–254
Dominique G, Tarascon J-M (1992) High-voltage-stable electrolytes for Li1+x Mn2O4/carbon secondary batteries. United States Patent US19920871855, 1993/03/09
Xu M, Liu Y, Li B, Li W, Li X, Hu S (2012) Tris (pentafluorophenyl) phosphine: an electrolyte additive for high voltage Li-ion batteries. Electrochem Commun 18:123–126
Manthiram A (2011) Materials challenges and opportunities of lithium ion batteries. J Phys Chem Lett 2(3):176–184. doi:10.1021/jz1015422
Zhang SS (2006) A review on electrolyte additives for lithium-ion batteries. J Power Sources 162(2):1379–1394. doi:10.1016/j.jpowsour.2006.07.074
Fergus JW (2010) Ceramic and polymeric solid electrolytes for lithium-ion batteries. J Power Sources 195(15):4554–4569
Girishkumar G, McCloskey B, Luntz AC, Swanson S, Wilcke W (2010) Lithium–air battery: promise and challenges. J Phys Chem Lett 1(14):2193–2203. doi:10.1021/jz1005384
Lewandowski A, Świderska-Mocek A (2009) Ionic liquids as electrolytes for Li-ion batteries—an overview of electrochemical studies. J Power Sources 194(2):601–609
CALSTART (2010) Energy storage compendium: batteries for electric and hybrid heavy duty vehicles. CALSTART, Pasadena
Jossen A, Weydanz W (2006) Moderne Akkumulatoren richtig einsetzen. Reichardt Verlag, Leipheim und München
Lee JH, Lee HM, Ahn S (2003) Battery dimensional changes occurring during charge/discharge cycles—thin rectangular lithium ion and polymer cells. J Power Sources 119–121:833–837
von Delft S, Gelhard C, Leker J (2012) Innovating in converging industries: challenges in the field of E-mobility. In: Paper presented at the Kraftwerk Batterie, Münster
Van den Bossche P, Vergels F, Van Mierlo J, Matheys J, Van Autenboer W (2006) SUBAT: an assessment of sustainable battery technology. J Power Sources 162(2):913–919
Acknowledgments
We thank Dr. René Schmitz for helpful discussions and comments on this project. Financial support from the German Research Foundation (DFG) and the NRW Graduate School of Chemistry is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wagner, R., Preschitschek, N., Passerini, S. et al. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J Appl Electrochem 43, 481–496 (2013). https://doi.org/10.1007/s10800-013-0533-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-013-0533-6