Abstract
A major challenge in the care of patients with heart failure and preserved ejection fraction (HFpEF) is the lack of proven therapies due to disappointing results from randomized controlled trials (RCTs). The heterogeneity of the HFpEF syndrome and the use of conventional RCT designs are possible reasons underlying the failure of these trials. There are several factors—including the widespread adoption of electronic health records, decreasing costs of obtaining high-dimensional data, and the availability of a wide variety of potential therapeutics—that have evolved to enable more innovative clinical trial designs in HFpEF. Here, we review the current landscape of HFpEF RCTs and present several innovative RCT designs that could be implemented in HFpEF, including enrichment trials, adaptive trials, umbrella trials, basket trials, and machine learning-based trials (including examples for each). Our hope is that the description of the aforementioned innovative trial designs will stimulate new approaches to clinical trials in HFpEF.




Similar content being viewed by others
References
Shah, S. J., Kitzman, D. W., Borlaug, B. A., van Heerebeek, L., Zile, M. R., Kass, D. A., et al. (2016). Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation, 134(1), 73–90.
Adamson, P. B., Abraham, W. T., Bourge, R. C., Costanzo, M. R., Hasan, A., Yadav, C., et al. (2014). Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circulation. Heart Failure, 7(6), 935–944.
Pfeffer, M. A., Claggett, B., Assmann, S. F., Boineau, R., Anand, I. S., Clausell, N., et al. (2015). Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation, 131(1), 34–42.
Pitt, B., Pfeffer, M. A., Assmann, S. F., Boineau, R., Anand, I. S., Claggett, B., et al. (2014). Spironolactone for heart failure with preserved ejection fraction. The New England Journal of Medicine, 370(15), 1383–1392.
Shah, S. J. (2017). Precision medicine for heart failure with preserved ejection fraction: an overview. J Cardiovasc Transl Res, (in press).
Shah, S. J. (2013). Matchmaking for the optimization of clinical trials of heart failure with preserved ejection fraction: no laughing matter. Journal of the American College of Cardiology, 62(15), 1339–1342.
Shah, S. J., Katz, D. H., & Deo, R. C. (2014). Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Failure Clinics, 10(3), 407–418.
Ford, I., & Norrie, J. (2016). Pragmatic trials. The New England Journal of Medicine, 375(5), 454–463.
Lund, L. H., Oldgren, J., & James, S. (2017). Registry-based pragmatic trials in heart failure: Current experience and future directions. Current Heart Failure Reports, 14(2), 59–70.
Senni, M., Paulus, W. J., Gavazzi, A., Fraser, A. G., Diez, J., Solomon, S. D., et al. (2014). New strategies for heart failure with preserved ejection fraction: The importance of targeted therapies for heart failure phenotypes. European Heart Journal, 35(40), 2797–2815.
Jonnalagadda, S. R., Adupa, A. K., Garg, R. P., Corona-Cox, J., & Shah, S. J. (2017). Text mining of the electronic health record: an information extraction approach for the automated identification and subphenotyping of HFpEF patients for clinical trials. Journal of Cardiovascular Translational Research, in press.
Website. http://www.pubmed.gov (search term: (hfpef [ti] OR “diastolic heart failure” [ti] OR “preserved ejection fraction” [ti]) AND review [pt])). Accessed 20 May 2017.
Butler, J., Fonarow, G. C., Zile, M. R., Lam, C. S., Roessig, L., Schelbert, E. B., et al. (2014). Developing therapies for heart failure with preserved ejection fraction: current state and future directions. JACC Heart Failure, 2(2), 97–112.
Senni, M., Greene, S. J., Butler, J., Fonarow, G. C., & Gheorghiade, M. (2017). Drug development for heart failure with preserved ejection fraction: what pieces are missing from the puzzle? The Canadian Journal of Cardiology, 33(6), 768–776.
Borlaug, B. A. (2014). The pathophysiology of heart failure with preserved ejection fraction. Nature Reviews. Cardiology, 11(9), 507–515.
Borlaug, B. A., & Paulus, W. J. (2011). Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. European Heart Journal, 32(6), 670–679.
Paulus, W. J., & Tschope, C. (2013). A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology, 62(4), 263–271.
Maurer, M. S., Elliott, P., Merlini, G., Shah, S. J., Waddington Cruz, M., Flynn, A., et al. (2017). Design and rationale of the phase 3 Tafamidis in ATTR-ACT (Transthyretin Cardiomyopathy Clinical Trial). Circulation. Heart Failure, in press.
Olivotto, I., Hellawell, J. L., Farzaneh-Far, R., Blair, C., Coppini, R., Myers, J., et al. (2016). Novel approach targeting the complex pathophysiology of hypertrophic cardiomyopathy: The impact of late sodium current inhibition on exercise capacity in subjects with symptomatic hypertrophic cardiomyopathy (LIBERTY-HCM) trial. Circulation. Heart Failure, 9(3), e002764.
Van Tassell, B. W., Buckley, L. F., Carbone, S., Trankle, C. R., Canada, J. M., Dixon, D. L., et al. (2017). Interleukin-1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D-HART2). Clinical Cardiology.
Biankin, A. V., Piantadosi, S., & Hollingsworth, S. J. (2015). Patient-centric trials for therapeutic development in precision oncology. Nature, 526(7573), 361–370.
Hollingsworth, S. J., & Biankin, A. V. (2015). The challenges of precision oncology drug development and implementation. Public Health Genomics, 18(6), 338–348.
Jurgensmeier, J. M., Eder, J. P., & Herbst, R. S. (2014). New strategies in personalized medicine for solid tumors: Molecular markers and clinical trial designs. Clinical Cancer Research, 20(17), 4425–4435.
Barzilai, N., Crandall, J. P., Kritchevsky, S. B., & Espeland, M. A. (2016). Metformin as a tool to target aging. Cell Metabolism, 23(6), 1060–1065.
Feldman, T., Komtebedde, J., Burkhoff, D., Massaro, J., Maurer, M. S., Leon, M. B., et al. (2016). Transcatheter interatrial shunt device for the treatment of heart failure: rationale and design of the randomized trial to REDUCE elevated left atrial pressure in heart failure (REDUCE LAP-HF I). Circulation. Heart Failure, 9(7).
Cleland, J. G., Tendera, M., Adamus, J., Freemantle, N., Polonski, L., Taylor, J., et al. (2006). The perindopril in elderly people with chronic heart failure (PEP-CHF) study. European Heart Journal, 27(19), 2338–2345.
Yusuf, S., Pfeffer, M. A., Swedberg, K., Granger, C. B., Held, P., McMurray, J. J., et al. (2003). Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet, 362(9386), 777–781.
Massie, B. M., Carson, P. E., McMurray, J. J., Komajda, M., McKelvie, R., Zile, M. R., et al. (2008). Irbesartan in patients with heart failure and preserved ejection fraction. The New England Journal of Medicine, 359(23), 2456–2467.
Bhatt, D. L., & Mehta, C. (2016). Adaptive designs for clinical trials. The New England Journal of Medicine, 375(1), 65–74.
Gallo, P., Chuang-Stein, C., Dragalin, V., Gaydos, B., Krams, M., Pinheiro, J., et al. (2006). Adaptive designs in clinical drug development—an executive summary of the PhRMA working group. Journal of Biopharmaceutical Statistics, 16(3), 275–283 discussion 285-291, 293-278, 311-272.
Hung, H. M., Wang, S. J., & O’Neill, R. (2007). Statistical considerations for testing multiple endpoints in group sequential or adaptive clinical trials. Journal of Biopharmaceutical Statistics, 17(6), 1201–1210.
Thall, P., Fox, P., & Wathen, J. (2015). Statistical controversies in clinical research: scientific and ethical problems with adaptive randomization in comparative clinical trials. Annals of Oncology, 26(8), 1621–1628.
Wang, S. J. (2010). Perspectives on the use of adaptive designs in clinical trials. Part I. Statistical considerations and issues. Journal of Biopharmaceutical Statistics, 20(6), 1090–1097.
Stallard, N., & Todd, S. (2011). Seamless phase II/III designs. Statistical Methods in Medical Research, 20(6), 623–634.
Bristow, M. R., Enciso, J. S., Gersh, B. J., Grady, C., Rice, M. M., Singh, S., et al. (2016). Detection and management of geographic disparities in the TOPCAT trial: lessons learned and derivative recommendations. JACC: Basic to Translational Science, 1(3), 180–189.
Pieske, B., Maggioni, A. P., Lam, C. S. P., Pieske-Kraigher, E., Filippatos, G., Butler, J., et al. (2017). Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. European Heart Journal, 38(15), 1119–1127.
Pieske, B., Butler, J., Filippatos, G., Lam, C., Maggioni, A. P., Ponikowski, P., et al. (2014). Rationale and design of the SOluble guanylate cyclase stimulatoR in heArT failurE studies (SOCRATES). European Journal of Heart Failure, 16(9), 1026–1038.
Solomon, S. D., Zile, M., Pieske, B., Voors, A., Shah, A., Kraigher-Krainer, E., et al. (2012). The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet, 380(9851), 1387–1395.
Wang, J., Chow, S. C., & Chang, M. (2016). Biomarker-driven adaptive Design for Precision Medicine. Journal of Translational Biomarkers & Diagnosis, 2, 15–24.
Shah, S. J., Cogswell, R., Ryan, J. J., & Sharma, K. (2016). How to develop and implement a specialized heart failure with preserved ejection fraction clinical program. Current Cardiology Reports, 18(12), 122.
Obokata, M., Reddy, Y. N., Pislaru, S. V., Melenovsky, V., & Borlaug, B. A. (2017). Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation.
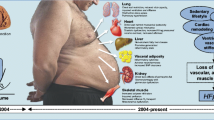
Kitzman, D. W., & Shah, S. J. (2016). The HFpEF obesity phenotype: the elephant in the room. Journal of the American College of Cardiology, 68(2), 200–203.
Meltzer, H. Y., & Roth, B. L. (2013). Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. The Journal of Clinical Investigation, 123(12), 4986–4991.
Shukla, A. P., Kumar, R. B., & Aronne, L. J. (2015). Lorcaserin Hcl for the treatment of obesity. Expert Opinion on Pharmacotherapy, 16(16), 2531–2538.
Kim, J., Moon, B. S., Lee, B. C., Lee, H. Y., Kim, H. J., Choo, H., et al. (2017). A potential PET radiotracer for the 5-HT2C receptor: synthesis and in vivo evaluation of 4-(3-[18F]fluorophenethoxy)pyrimidine. ACS Chemical Neuroscience, 8(5), 996–1003.
Lim, S. L., Lam, C. S., Segers, V. F., Brutsaert, D. L., & De Keulenaer, G. W. (2015). Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. European Heart Journal, 36(31), 2050–2060.
Crea, F., Bairey Merz, C. N., Beltrame, J. F., Kaski, J. C., Ogawa, H., Ong, P., et al. (2017). The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. European Heart Journal, 38(7), 473–477.
Lam, C. S., & Lund, L. H. (2016). Microvascular endothelial dysfunction in heart failure with preserved ejection fraction. Heart, 102(4), 257–259.
Giamouzis, G., Schelbert, E. B., & Butler, J. (2016). Growing evidence linking microvascular dysfunction with heart failure with preserved ejection fraction. Journal of the American Heart Association, 5(2).
Bairey Merz, C. N., Pepine, C. J., Walsh, M. N., & Fleg, J. L. (2017). Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation, 135(11), 1075–1092.
Desai, C. S., Lee, D. C., & Shah, S. J. (2011). Systemic sclerosis and the heart: current diagnosis and management. Current Opinion in Rheumatology, 23(6), 545–554.
Deo, R. C. (2015). Machine learning in medicine. Circulation, 132(20), 1920–1930.
Mnih, V., Kavukcuoglu, K., Silver, D., Rusu, A. A., Veness, J., Bellemare, M. G., et al. (2015). Human-level control through deep reinforcement learning. Nature, 518(7540), 529–533.
Zeevi, D., Korem, T., Zmora, N., Israeli, D., Rothschild, D., Weinberger, A., et al. (2015). Personalized nutrition by prediction of glycemic responses. Cell, 163(5), 1079–1094.
Silver, D., Huang, A., Maddison, C. J., Guez, A., Sifre, L., van den Driessche, G., et al. (2016). Mastering the game of go with deep neural networks and tree search. Nature, 529(7587), 484–489.
Zhao, Y., Kosorok, M. R., & Zeng, D. (2009). Reinforcement learning design for cancer clinical trials. Statistics in Medicine, 28(26), 3294–3315.
Zhao, Y., Zeng, D., Socinski, M. A., & Kosorok, M. R. (2011). Reinforcement learning strategies for clinical trials in nonsmall cell lung cancer. Biometrics, 67(4), 1422–1433.
Trusheim, M. R., Shrier, A. A., Antonijevic, Z., Beckman, R. A., Campbell, R. K., Chen, C., et al. (2016). PIPELINEs: creating comparable clinical knowledge efficiently by linking trial platforms. Clinical Pharmacology and Therapeutics, 100(6), 713–729.
Ma, J., Hobbs, B. P., & Stingo, F. C. (2015). Statistical methods for establishing personalized treatment rules in oncology. BioMed Research International, 2015, 670691.
Rothwell, P. M. (2005). Treating individuals 2. Subgroup analysis in randomised controlled trials: Importance, indications, and interpretation. Lancet, 365(9454), 176–186.
Burke, J. F., Sussman, J. B., Kent, D. M., & Hayward, R. A. (2015). Three simple rules to ensure reasonably credible subgroup analyses. BMJ, 351, h5651.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Dr. Shah is supported by the National Institutes of Health R01 HL107577 and R01 HL127028 and American Heart Association No. 16SFRN28780016 and No. 15CVGPSD27260148.
Conflict of Interest
Dr. Shah has received research grants from Actelion, AstraZeneca, Corvia, and Novartis and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Ironwood, Merck, Novartis, Sanofi, and United Therapeutics.
Ethical Approval
This review article does not contain any primary data from studies with human participants or animals performed by the author.
Additional information
Editor Enrique Lara-Pezzi oversaw the review of this article
Rights and permissions
About this article
Cite this article
Shah, S.J. Innovative Clinical Trial Designs for Precision Medicine in Heart Failure with Preserved Ejection Fraction. J. of Cardiovasc. Trans. Res. 10, 322–336 (2017). https://doi.org/10.1007/s12265-017-9759-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-017-9759-8