Abstract
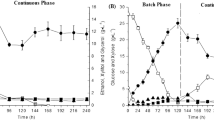
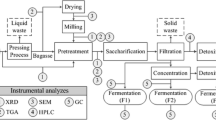
There is an increasing worldwide interest in bioethanol production from agricultural, industrial, and urban residues for both ecological and economic reasons. The acid hydrolysis of cassava pulp to reducing sugars and their fermentation to ethanol were evaluated in a fibrousbed bioreactor with immobilized Δldh, a genetically engineered Thermoanaerobacterium aotearoense. A maximum yield of total reducing sugars of 53.5% was obtained after 8 h of hydrolysis at 85oC in 0.4 mol/L hydrochloric acid with a solid-to-liquid ratio of 1:20, which was optimized by using an orthogonal design based on preliminary experiments. In the FBB, the fed-batch fermentation, using glucose as the sole carbon source, gave a maximum ethanol production of 38.3 g/L with a yield of 0.364 g/g in 100 h; whereas the fed-batch fermentation, using xylose as the sole carbon source, gave 34.1 g/L ethanol with a yield of 0.342 g/g in 135 h. When cassava pulp hydrolysate was used as a carbon source, 39.1 g/L ethanol with a yield of 0.123 g/g cassava pulp in185 h was observed, using the fed-batch fermentation model. In addition, for repeated batch fermentation of cassava pulp hydrolysate carried out in the fibrous-bed bioreactor, long-term operation with high ethanol yield and volumetric productivity were achieved. The above results show that the acid hydrolysate of cassava pulp can be used for ethanol production in a fibrous-bed bioreactor, although some inhibition phenomena were observed during the process of fermentation.
Similar content being viewed by others
References
Helena, H. L. and P. O. Ralph (2001) Biomass and renewable fuel. Fuel. Bioproc. Technol. 71: 187–195.
Katahira, S., M. Ito, H. Takema, Y. Fujita, T. Tanino, T. Tanaka, H. Fukuda, and A. Kondo (2008) Improvement of ethanol productivity during xylose and glucose co-fermentation by xyloseassimilating S. cerevisiae via expression of glucose transporter Sut1. Enz. Microb. Technol. 43: 115–119.
Lin, Y. and S. H. Tanaka (2006) Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 69: 627–642.
Prasad, S., A. Singh, and H. C. Joshi (2007) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recy. 50: 1–39.
Karimi, K., G. Emtiazi, and M. J. Taherzadeh (2006) Ethanol production from dilute-acid pretreated rice straw by simultaneous saccharification and fermentation with Mucor indicus, Rhizopus oryzae, and Saccharomyces cerevisiae. Enz. Microb. Technol. 40: 138–144.
Kumar, A., L. K. Singh, and S. Ghosh (2009) Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Bioresour. Technol. 100: 3293–3297.
Li, H., N. J. Kim, M. Jiang, J. W. Kang, and H. N. Chang (2009) Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour. Technol. 100: 3245–3251.
Georgieva, T. I., M. J. Mikkelen, and B. K. Ahring (2008) Ethanol production from wet-exploded wheat straw hydrolysate by thermophilic anaerobic bacterium Thermoanaerobacter BG1L1 in a continuous immobilized reactor. Appl. Biochem. Biotechnol. 145: 99–110.
Dennis, J. B., E. S. Gerard, J. K. Michael, and C. J. James (2004) Ethanol recovery from corn fiber hydrolysate fermentations by pervaporation. Bioresour. Technol. 92: 15–19.
Lin, C. W., C. H. Wu, D. T. Tran, M. C. Shih, W. H. Li, and C. F. Wu (2011) Mixed culture fermentation from lignocellulosic materials using thermophilic lignocellulose-degrading anaerobes. Proc. Biochem. 46: 489–493.
Lin, C. Y. and W. C. Hung (2008) Enhancement of fermentative hydrogen/ethanol production from cellulose using mixed anaerobic cultures. Int. J. Hydrogen. Energ. 33: 3660–3667.
Sriroth, K., R. Chollakup, S. Chotineeranat, K. Piyachomkwan, and C. G. Oates (2000) Processing of cassava waste for improved biomass utilization. Bioresour. Technol. 71: 63–69.
Kosugi, A., A. Kondo, M. Ueda, Y. Murata, P. Vaithanomsat, W. Thanapase, T. Arai, and Y. Mori (2009) Production of ethanol from cassava pulp via fermentation with a surface-engineered yeast strain displaying glucoamylase. Renew. Energ. 34: 1354–1358.
Li, S., C. F. Lai, Y. H. Cai, X. F. Yang, S. Yang, M. J. Zhu, J. F. Wang, and X. N. Wang (2010) High efficiency hydrogen production from glucose/xylose by the ldh-deleted Thermoanaerobacterium strain. Bioresour. Technol. 101: 8718–8724.
Cai, Y. H., C. F. Lai, S. Li, Z. X. Liang, M. J. Zhu, S. Z. Liang, and J. F. Wang (2011) Disruption of lactate dehydrogenase through homologous recombination to improve bioethanol production in Thermoanaerobacterium aotearoense. Enz. Microb. Technol. 48: 155–161.
Aristidou, A. and M. Penttila (2000) Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotech. 11: 187–198.
Lynd, L. R., P. J. Weimer, W. H. Zyl, and I. S. Pretorius (2002) Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. R. 66: 506–577.
Liu, C. Z., F Wang, and F. O. Yang (2009) Ethanol fermentation in a magnetically fluidized bed reactor with immobilized Saccharomyces cerevisiae in manetic particles. Bioresour. Technol 100: 878–882.
Silva, E. M. and S. T. Yang (1995) Kinetics and stability of a fibrous-bed bioreactor for continuous production of lactic acid from unsupplemented acid whey. J. Biotechnol. 41: 59–70.
Jiang, L., J. F. Wang, S. Z. Liang, X. N. Wang, P. L. Cen, and Z. N. Xu (2009) Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour. Technol. 100: 3403–3409.
Jiang, L., J. F. Wang, S. Z. Liang, X. N. Wang, P. L. Cen, and Z. N. Xu (2010) Production of butyric acid from glucose and xylose with immobilized cells of Clostridium tyrobutyricum in a fibrousbed bioreactor. Appl. Biochem. Biotech. 160: 350–359.
Jiang, L., J. F. Wang, S. Z. Liang, J. Cai, Z. N. Xu, P. L. Cen, S. T. Yang, and S. Li (2011) Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum in a fibrous bed bioreactor. Biotechnol. Bioeng. 108: 31–40.
Huang, Y. L., K. Mann, J. M. Novak, and S. T. Yang (1998) Acetic acid production from fructose by Clostridium formicoaceticum immobilized in a fibrous-bed bioreactor. Biotechnol. Progr. 14: 800–806.
Suwannakham, S. and S. T. Yang (2005) Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnol. Bioeng. 91: 325–337.
Huang, W. C., D. E. Ramey, and S. T. Yang (2004) Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed bioreactor. Appl. Biochem. Biotech. 115: 887–898.
Ozkan, M., E. I. Yilmaz, L. R. Lynd, and G. Ozcengiz (2004) Cloning and expression of the Clostridium thermocellum L-lactate dehydrogenase gene in Escherichia coli and enzyme characterization. Can. J. Microbiol. 50: 845–851.
Sun, R., J. M. Lawther, and W. B. Banks (1996) Fractional and structural characterization of wheat straw hemicellulose. Carbohyd. Polym. 29: 325–331.
Cai, Y. H., C. F. Lai, Z. X. Liang, P. Li, J. F. Wang, M. J. Zhu, and S. Z. Liang (2010) Fermentative production of ethanol affected by deleting lactate dehydrogenase from Thermoanaerobacterium aotearoense. J. South China Univ. Technol. 38: 140–144.
Author information
Authors and Affiliations
Corresponding author
Additional information
These two author’s made equal contribution to this paper.
Rights and permissions
About this article
Cite this article
Cai, YH., Liang, ZX., Li, S. et al. Bioethanol from fermentation of cassava pulp in a fibrous-bed bioreactor using immobilized Δldh, a genetically engineered Thermoanaerobacterium aotearoense . Biotechnol Bioproc E 17, 1270–1277 (2012). https://doi.org/10.1007/s12257-012-0405-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-012-0405-7