Abstract
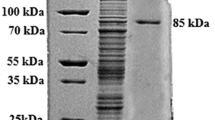
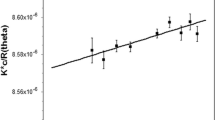
A thermotolerant fungus identified as Aspergillus niveus was isolated from decomposing materials and it has produced excellent levels of hydrolytic enzymes that degrade plant cell walls. A. niveus germinated faster at 40 °C, presenting protein levels almost twofold higher than at 25 °C. The crude extract of the A. niveus culture was purified by diethylaminoethyl (DEAE)-cellulose, followed by Biogel P-100 column. Polygalacturonase (PG) is a glycoprotein with 37.7 % carbohydrate, molecular mass of 102.6 kDa, and isoelectric point of 5.4. The optimum temperature and pH were 50 °C and 4.0–6.5, respectively. The enzyme was stable at pH 3.0 to 9.0 for 24 h. The DEAE-cellulose derivative was about sixfold more stable at 60 °C than the free enzyme. Moreover, the monoaminoethyl-N-aminoethyl-agarose derivative was tenfold more stable than the free enzyme. PG was 232 % activated by Mn2+. The hydrolysis product of sodium polypectate corresponded at monogalacturonic acid, which classifies the enzyme as an exo-PG. The K m, V max, K cat, and K cat/K m values were 6.7 mg/ml, 230 U/mg, 393.3/s, and 58.7 mg/ml/s, respectively. The N-terminal amino acid sequence presented 80 % identity with PglB1, PglA2, and PglA3 putative exo-PG of Aspergillus fumigatus and an exo-PG Neosartorya fischeri.





Similar content being viewed by others
References
Betini JHA, Michelin M, Peixoto-Nogueira SC, Jorge JA, Terenzi HF, Polizeli MLTM (2009) Xylanases from Aspergillus niger, Aspergillus niveus and Aspergillus ochraceus produced under solid-state fermentation and their application in cellulose pulp bleaching. Bioprocess Biosyst Eng 32:819–824. doi:10.1007/s00449-009-0308-y
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant-proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
da Silva TM, Maller A, Damásio AR, Michelin M, Ward RJ, Hirata IY, Jorge JA, Terenzi HF, de Polizeli ML (2009) Properties of a purified thermostable glucoamylase from Aspergillus niveus. J Ind Microbiol Biotechnol 36:1439–1446. doi:10.1007/s10295-009-0630-z
Damásio ARD, da Silva TM, Maller A, Jorge JA, Terenzi HF, Polizeli MDTD (2010) Purification and partial characterization of an exo-polygalacturonase from Paecilomyces variotii liquid cultures. Appl Biochem Biotechnol 160:1496–1507. doi:10.1007/s12010-009-8682-0
Davis BJ (1964) Disc electrophoresis: method and application to human serum proteins. Ann N Y Acad Sci 121:404–427
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Fahmy AS, El-beih FM, Mohamed SA, Abdel-Gany SS, Abd-Elbaky EA (2008) Characterization of an exopolygalacturonase from Aspergillus niger. Appl Biochem Biotecnol 149:205–217. doi:10.1007/s12010-007-8107-x
Fernandez-Lafuente R, Rosell CM, Rodriguez V, Santana C, Soler G, Bastida A, Guisan JM (1993) Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb Technol 15:546–550
Gomes E, Iembo T, da Silva R (2001) Production, characterization and properties of polysaccharide depolymerizing enzymes from a strain of Curvularia inaequalis. Folia Microbiol 46:303–308
Gomes E, Leite RS, da Silva R, Silva D (2009) Purification of an exopolygalacturonase from Penicillium viridicatum RFC3 produced in submerged fermentation. Int J Microbiol 2009:631942. doi:10.1155/2009/631942
Hanes CS (1932) Studies on plant amylases. I. The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J 26:1406–1421
Jurick WM, Vico I, Gaskins VL, Garrett WM, Whitaker BD, Janisiewicz WJ, Conway WS (2010) Purification and biochemical characterization of polygalacturonase produced by Penicillium expansum during postharvest decay of ‘Anjou’ pear. Phytopathol 100:42–48. doi:10.1094/Phyto-100-1-0042
Kashyap DR, Vohra PK, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77:215–227
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685
Maller A, Damásio ARL, Silva TM, Jorge JA, Terenzi HF, Polizeli MLTM (2011) Biotechnological potential of agro-industrial wastes as a carbon source to thermostable polygalacturonase production in Aspergillus niveus. Enzyme Res 2011:289206. doi:10.4061/2011/289206
Martins ES, Silva D, Leite RSR, Gomes E (2007) Purification and characterization of polygalacturonase produced by thermophilic Thermoascus aurantiacus CBMAI-756 in submerged fermentation. Anton Leeuw Int J G 91:291–299. doi:10.1007/s10482-006-9114-6
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Reversible enzyme immobilization via a very strong and nondistorting ionic adsorption on support-polyethylenimine composites. Biotechnol Bioeng 68:98–105
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
O'Farrell PZ, Goodman HM, O'Farrell PH (1977) High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133–1141
Pedrolli DB, Carmona EC (2010) Purification and characterization of the exopolygalacturonase produced by Aspergillus giganteus in submerged cultures. J Ind Microbiol Biotechnol 37:567–573
Peixoto-Nogueira SD, Michelin M, Betini JHA, Jorge JA, Terenzi HF, Polizeli MDTD (2009) Production of xylanase by Aspergilli using alternative carbon sources: application of the crude extract on cellulose pulp biobleaching. J Ind Microbiol Biotechnol 36:149–155. doi:10.1007/s10295-008-0482-y
Rizzatti AC, Sandrim VC, Jorge JA, Terenzi HF, Polizeli MLTM (2004) Influence of temperature on the properties of the xylanolytic enzymes of the thermotolerant fungus Aspergillus phoenicis. J Ind Microbiol Biotechnol 31:88–93. doi:10.1007/s10295-004-0120-2
Round AN, Rigby NM, MacDougall AJ, Morris VJ (2010) A new view of pectin structure revealed by acid hydrolysis and atomic force microscopy. Carbohydr Res 345:487–497. doi:10.1016/j.carres.2009.12.019
Thakur A, Pahwa R, Singh S, Gupta R (2010) Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Res 2010:170549
Wiseman A (1975) Handbook of enzyme biotechnology. Wiley, London
Zeni J, Cence K, Grando CE, Tiggermann L, Colet R, Lerin LA, Cansian RL, Toniazzo G, de Oliveira D, Valduga E (2011) Screening of pectinase-producing microorganisms with polygalacturonase activity. Appl Biochem Biotecnol 163:383–392. doi:10.1007/s12010-010-9046-5
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho de Desenvolvimento Científico e Tecnológico (CNPq). J.A.J. and M.L.T.M.P. are research fellows of CNPq. A.M., A.R.L.D., and T.M.S. were recipients of a FAPESP Fellowship. We thank Dr. Dalton Amorim and Diego Fachin for the image support and Ricardo Alarcon and Mauricio de Oliveira for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maller, A., da Silva, T.M., Damásio, A.R.d.L. et al. Functional properties of a manganese-activated exo-polygalacturonase produced by a thermotolerant fungus Aspergillus niveus . Folia Microbiol 58, 615–621 (2013). https://doi.org/10.1007/s12223-013-0249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-013-0249-3