Abstract
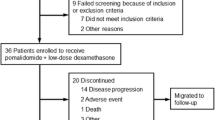
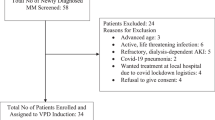
Determinants of the efficacy and safety of pomalidomide (POM) monotherapy or POM plus dexamethasone (DEX) (POM/DEX) for relapsed and refractory multiple myeloma (RRMM) were examined retrospectively in a real-world clinical practice setting in Japan. The subjects were 108 patients registered with the Kansai Myeloma Forum, who were treated with either POM or POM/DEX. Of these, 79 (73%), 73 (68%), and 58 (54%) were resistant to bortezomib (BTZ), lenalidomide (LEN), and both BTZ and LEN, respectively. The median overall survival (OS) was not reached. The median time to treatment failure (TTF) was 4.4 months. The best response was recorded in 96 patients, with a 31% overall response rate (ORR) and a 79% rate of achieving at least stable disease. Number of pre-POM regimens ≥ 5, non-IgG-type M-protein, and time from initial therapy to POM or POM/DEX therapy < 2 years were associated with shorter TTF and OS. Frequent (> 10%) severe adverse events included neutropenia (55.1%), thrombocytopenia (33.7%), anemia (30.6%), febrile neutropenia (12.2%), fatigue (11.2%), and anorexia (10.2%). In conclusion, POM and POM/DEX showed substantial efficacy against RRMM, but new combination therapies with POM are needed to improve efficacy further without causing hematologic toxicities.



Similar content being viewed by others
References
Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30:1005–17.
Sheng Z, Liu G. Pooled analysis of the reports of pomalidomide after failure of lenalidomide and (or) bortezomib for multiple myeloma. Hematol Oncol. 2016;34:102–7.
Fouquet G, Pegourie B, Macro M, Petillon MO, Karlin L, Caillot D, et al. Safe and prolonged survival with long-term exposure to pomalidomide in relapsed/refractory myeloma. Ann Oncol. 2016;27:902–7.
Shi CX, Kortum KM, Zhu YX, Jedlowski P, Bruins L, Braggio E, et al. Proteasome inhibitors block Ikaros degradation by lenalidomide in multiple myeloma. Haematologica. 2015;100:e315–7.
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN). Br J Haematol. 2014;164:811–21.
Iida S. Mechanisms of action and resistance for multiple myeloma novel drug treatments. In t J Hematol. 2016;104:271–2.
Ito T, Handa H. Cereblon and its downstream substrates as molecular target of immunomodulatory drugs. Int J Hematol. 2016;104:293–9.
Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, et al. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968–75.
San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–66.
Sriskandarajah P, Pawlyn C, Mohammed K, Dearden CE, Davies FE, Morgan GJ, et al. The efficacy and tolerability of pomalidomide in relapsed/refractory myeloma patients in a “real-world” study: the Royal Marsden Hospital experience. Leuk Lymphoma. 2017;58:494–7.
Matsue K, Iwasaki H, Chou T, Tobinai K, Sunami K, Ogawa Y, et al. Pomalidomide alone or in combination with dexamethasone in Japanese patients with refractory or relapsed and refractory multiple myeloma. Cancer Sci. 2015;106:1561–7.
Ichinohe T, Kuroda Y, Okamoto S, Matsue K, Iida S, Sunami K, et al. A multicenter phase 2 study of pomalidomide plus dexamethasone in patients with relapsed and refractory multiple myeloma: the Japanese MM-011 trial. Exp Hematol Oncol. 2015;5:11.
Kobayashi T, Kuroda J, Fuchida S, Kaneko H, Yagi H, Shibayama H, et al. Impact of early use of lenalidomide and low-dose dexamethasone on clinical outcomes in patients with relapsed/refractory multiple myeloma. Int J Hematol. 2015;101:37–45.
Kuroda J, Shimura Y, Ohta K, Tanaka H, Shibayama H, Kosugi S, et al. Limited value of the international staging system for predicting long-term outcome of transplant-ineligible, newly diagnosed, symptomatic multiple myeloma in the era of novel agents. Int J Hematol. 2014;99:441–9.
Durie BG, Harousseau JL, Saqn Miguel JF, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Greipp PR, San Miguel J, Durie BG, Crowly JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical sstatistics. Bone Marrow Transplant. 2013;48:452–8.
Palumbo A, Mateos MV, Bringhen S, San Miguel J. Practical management of adverse events in multiple myeloma: can therapy be attenuated in older patients? Blood Rev. 2011;25:181–91.
Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23.
Lagana AS, Sofo V, Vitale SG, Triolo O. Epithelial ovarian cancer inherent resistance: may the pleiotropic interaction between reduced immunosurveillance and drug-resistant cells play a key role? Gynecol Oncol Rep. 2016;18:57–8.
Yang WC, Lin SF. Mechanisms of drug resistance in relapse and refractory multiple myeloma. Biomed Res Int 2015; 2015:341430.
Fakhri B, Vij R. Clonal Evolution in Multiple Myeloma. Clin Lymphoma Myeloma Leuk. 2016. https://doi.org/10.1016/j.clml.2016.02.025.
Raza S, Safyan RA, Rosenbaum E, Bowman AS, Lentzsch S. Optimizing current and emerging therapies in multiple myeloma: a guide for the hematologist. Ther Adv Hematol. 2017;8:55–70.
Suehara Y, Takamatsu H, Fukumoto K, Fujisawa M, Narita K, Usui Y, et al. Abnormal heavy/light chain ratio after treatment is associated with shorter survival in patients with IgA myeloma. Cancer Sci. 2017;108:187–92.
Wang GR, Sun WJ, Chen WM, Huang ZX, Zhang JJ, An N, et al. Immunoglobulin D Multiple Myeloma: disease Profile, Therapeutic Response, and Survival. Acta Haematol. 2016;136:140–6.
Dimopoulos MA, Weisel KC, Song KW, Delforge M, Karlin L, Goldschmidt H, et al. Cytogenetics and long-term survival of patients with refractory or relapsed and refractory multiple myeloma treated with pomalidomide and low-dose dexamethasone. Haematologica. 2015;100:1327–33.
Yu W, Li J, Chen L. Prognostic value and efficacy evaluation of novel drugs for cytogenetic aberrations in multiple myeloma: a meta-analysis. Int J Clin Exp Med. 2014;7:4051–62.
Leleu X, Karlin L, Macro M, Hulin C, Garderet L, Roussel M, et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): iFM 2010-02 trial results. Blood. 2015;125:1411–7.
Zou Y, Ma X, Yu H, Hu C, Fan L, Ran X. Carfilzomib/pomalidomide single-agent or in combination with other agents for the management of relapsed/refractory multiple myeloma: a meta-analysis of 37 trials. Oncotarget. 2017;8:39805–17.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621–31.
Lonial S, Weiss BM, Usmani S, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–60.
Hanaizi Z, Flores B, Hemmings R, Camarero J, Sancho-Lopez A, Salmonson T, et al. The European medicines agency review of pomalidomide in combination with low-dose dexamethasone for the treatment of adult patients with multiple myeloma: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2015;20:329–34.
Dimopoulos MA, Palumbo A, Corradini P, Cavo M, Delforge M, Di Raimondo F, et al. Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): a phase 3b study in refractory multiple myeloma. Blood. 2016;128:497–503.
Siegel DS, Schiller GJ, Song KW, Agajanian R, Stockerl-Goldstein K, Kaya H, et al. Pomalidomide + low-dose dexamethasone following second-line lenalidomide-based therapy in relapsed or refractory multiple myeloma: a phase 2 study investigating efficacy and safety. Blood. 2016;128:4497.
Acknowledgements
We wish to thank all myeloma patients registered in KMF and all KMF investigators for their scientific support. We also thank Ms. Okuyama R for her assistance to this study.
Author information
Authors and Affiliations
Consortia
Contributions
Author contributions
YMK and JK designed the study. YMK and IY performed the statistical analysis. HK, YK, SF, AN, HS, NU, HU, HY, SK, TM, JI, MM, KO, MI, HK, MK, KW, CS, SN, KI, MH, IM, YK, and ATK provided patient data.
Corresponding author
Ethics declarations
Conflict of interest
This work was supported by grants from Celgene Co., Ltd., Takeda Pharmaceutical Co., Ltd., Fujimoto Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Matsumura-Kimoto, Y., Kuroda, J., Kaneko, H. et al. Pomalidomide with or without dexamethasone for relapsed/refractory multiple myeloma in Japan: a retrospective analysis by the Kansai Myeloma Forum. Int J Hematol 107, 541–550 (2018). https://doi.org/10.1007/s12185-018-2416-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-018-2416-4