Abstract
Background
The immunomodulatory agent pomalidomide in combination with low-dose dexamethasone has demonstrated efficacy and safety for the treatment of relapsed and refractory multiple myeloma (RRMM) in phase 2 and 3 trials. However, these trials enrolled very few Asian patients.
Methods
This phase 2 study investigated pomalidomide plus low-dose dexamethasone in 36 Japanese patients with RRMM after ≥2 prior therapies.
Results
Patients enrolled in the study had a relatively high disease burden (81 % Durie–Salmon stage II or III) and were heavily pretreated (median, 6.5 prior antimyeloma regimens). The overall response rate was 42 % (1 patient with complete response and 14 with partial response), with an additional 44 % (16 patients) achieving stable disease (SD). Response rates in patients aged ≤65 years and >65 years were 47 and 35 %, respectively. None of the five patients with extramedullary disease achieved a response, with three of them maintaining SD of short duration. Median progression-free survival was 10.1 months after a 7.7-month median follow-up, and the median overall survival was not reached. The most frequent grade ≥3 adverse events (AEs) were neutropenia (64 %), anemia (42 %), and thrombocytopenia (31 %). The most frequent nonhematologic grade ≥3 AEs were pneumonia and decreased appetite (8 % each). Adverse events in patients aged >65 years were similar to those in patients aged ≤65 years, except for a higher rate of grade ≥3 pneumonia.
Conclusions
Collectively, the results of this study demonstrate that pomalidomide plus low-dose dexamethasone is an effective and safe treatment for Japanese patients with RRMM, although careful attention needs to be paid to serious infections.
Trial registration: Clinicaltrials.gov NCT02011113
Similar content being viewed by others
Background
Although the introduction of thalidomide, lenalidomide, and bortezomib has improved the survival of patients with multiple myeloma (MM) [1], MM remains incurable, and median overall survival (OS) for patients who have become refractory to bortezomib and thalidomide or lenalidomide is only 9 months [2]. Pomalidomide is a distinct IMiD® immunomodulatory compound with a mechanism of action that includes tumoricidal, immunomodulatory, and antiangiogenic effects [3]. In combination with low-dose dexamethasone, pomalidomide was approved in the United States, Canada, and the European Union for the treatment of patients with relapsed and refractory MM (RRMM) who have received ≥2 prior therapies, including lenalidomide and bortezomib, and who have demonstrated disease progression on the last therapy (United States, Canada, European Union) or within 60 days of completion of the last therapy (United States) [4–6]. In addition, this regimen has recently been approved for the treatment of Japanese patients with RRMM.
In a North American phase 1/2 RRMM study (MM-002), pomalidomide plus low-dose dexamethasone significantly extended progression-free survival (PFS) compared with pomalidomide alone [7, 8]. Furthermore, the regimen significantly improved both PFS and OS compared with high-dose dexamethasone alone in an international phase 3 trial (MM-003) [9]. The safety profile of pomalidomide plus low-dose dexamethasone was acceptable in both studies [8, 9]. However, the number of Asian patients who were enrolled in these previous studies was very small. Additionally, there are no phase 2 studies demonstrating the efficacy and safety profile of pomalidomide in Asian patients with RRMM.
Because pharmacokinetic (PK) and safety profiles of a drug can be affected by ethnicity [10–12], the phase 1 MM-004 study evaluated the tolerated dose, PK, safety, and efficacy of pomalidomide, alone and in combination with low-dose dexamethasone, in Japanese patients with RRMM [13]. Pomalidomide 4 mg/day, the recommended dose in the United States, Canada, and European Union [4–6], was identified as the tolerated dose in this patient population [13], consistent with previous observations in Caucasian patients [7]. These results led us to the phase 2 study, which investigated the efficacy and safety of pomalidomide plus low-dose dexamethasone in Japanese patients with RRMM.
Results
Patient characteristics
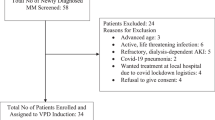
A total of 36 patients were enrolled between December 2013 and July 2014 at 13 sites in Japan; all patients were of Asian origin (Fig. 1). The median age was 64.5 years, and 11 % of the patients were aged >75 years (Table 1). The median time from first diagnosis was 4.7 years. Five patients (14 %) presented with extramedullary plasmacytoma in bone (n = 4) and liver (n = 1). Patients had a relatively high disease burden, including Durie–Salmon stage II or III disease in 81 %, and were heavily pretreated, with a median of 6.5 prior antimyeloma regimens. All but 1 patient (97 %) were refractory to lenalidomide, and 58 % were refractory to both lenalidomide and bortezomib.
Study treatment
Median treatment duration was 5.5 months (range, 0.3–12.0 months), and the median number of treatment cycles was 6 (range, 1–13 cycles). At the data cutoff (February 3, 2015), 16 patients (44 %) remained on the protocol treatment. Disease progression was the most common reason for discontinuation (14 patients, 39 %). Three patients (8 %) discontinued because of an adverse event (AE), including 1 fatal AE of aggravated asthma and pneumonia, and three patients (8 %) discontinued for other reasons (Fig. 1).
Efficacy
All 36 patients received study treatment and were evaluable for efficacy. The overall response rate (ORR) was 42 % (15 patients; 95 % CI, 26–58 %), with 1 patient (3 %) achieving a complete response (CR) and 14 patients (39 %) achieving a partial response (PR; Table 2). Stable disease (SD) was recorded as the best response in 16 patients (44 %). Of these 36 evaluable patients, final pomalidomide doses at the last follow-up were 4 mg in 27 patiens, 3 mg in seven patients, and 2 mg in two patients with ORR of 44 % (12/27 patients, 1 CR and 11 PRs), 43 % (3/7 patients, all PRs) and 0 % in each dose group, respectively (Table 3). The median time to first response was 1.9 months, including 2 patients whose response improved from SD after ≥4 cycles of treatment (Fig. 2). The median duration of response (DOR) was not reached (95 % CI, 4.6 months-not estimable). After a median follow-up of 7.7 months, the median PFS was 10.1 months (Fig. 3). A prespecified final OS analysis was conducted using a data cutoff of September 25, 2015; after median follow-up of 11.3 months, the 1-year OS was 58.5 %.
In patients aged ≤65 years, the ORR was 47 % (9/19 patients, all PRs), and in patients aged >65 years, an ORR of 35 % was observed (6/17 patients, 1 CR and 5 PRs). One of four patients aged >75 years achieved a PR. Analysis of impact of disease stage at the time of protocol enrollment showed that ORR in Durie–Salmon stage III disease (23 %) tended to be lower than that in stage I (57 %) or stage II (50 %) disease, although it is not statistically significant (P = 0.28). ORR was 43 % among patients who were refractory to lenalidomide (15/35 patients, 1 CR and 14 PRs) and 33 % among those refractory to both lenalidomide and bortezomib (7/21 patients, all PRs). Recent studies have shown that thalidomide is effective in patients refractory to bortezomib and lenalidomide treatment [14, 15]. ORR among patients who had previously received thalidomide and those who did not receive thalidomide was 33 % (4/12 patients, all PRs) and 46 % (11/24 patients, with 1 CR and 10 PRs), respectively, with no significant difference between two groups (P = 0.72), although the latter showed a trend toward longer PFS (3.3 months versus not reached, P = 0.21). Median OS was not reached for patients who received or did not receive prior thalidomide. Of the five patients with plasmacytomas, none achieved a response, with SD recorded as the best response in three patients. The median PFS for these five patients was 1.8 months.
Safety
All 36 patients reported ≥1 AE, and 31 patients (86 %) experienced a grade ≥3 AE, regardless of causality (Table 4). The most frequently reported grade ≥3 hematologic AEs regardless of causality were neutropenia (23 patients, 64 %), anemia (15 patients, 42 %), and thrombocytopenia (11 patients, 31 %). The most frequently reported grade ≥3 nonhematologic AEs regardless of causality were pneumonia (three patients, 8 %) and decreased appetite (three patients, 8 %). Other frequently reported AEs (any grade) were pyrexia (nine patients, 25 %); nasopharyngitis (eight patients, 22 %); and gastrointestinal disorders, including constipation (eight patients, 22 %), diarrhea, and nausea (seven patients, 19 % each).
Peripheral neuropathy (PN) of any grade occurred in three patients (8 %) and was considered to be treatment-related in all cases. No occurrences of deep vein thrombosis or pulmonary embolism were reported; all patients received thromboprophylaxis, most commonly with aspirin (94 % of patients). Febrile neutropenia was observed in one patient (3 %), and severe infections and infestations occurred in three patients (8 %). Serious AEs were reported in 13 patients (36 %) and were considered treatment related in six patients (17 %). Constipation and pneumonia were the only 2 treatment-related serious AEs that occurred in >1 patient (two patients, 6 % each).
Pomalidomide dosing was interrupted in 15 patients (42 %) and reduced in nine patients (25 %) due to AEs. AEs leading to dose reductions in >1 patient were thrombocytopenia (three patients, 8 %), anemia (two patients, 6 %), and leukopenia (two patients, 6 %). Three patients (8 %) had ≥1 AE that led to discontinuation of study treatment, all of which were considered treatment related. AEs leading to discontinuation were asthma, dyspnea, pleural effusion, anemia, pyrexia, and pneumonia. Nine patients (25 %) died during the study; eight deaths were due to progression of MM, and one was due to an AE of pneumonia and aggravated asthma that was suspected to be related to study treatment.
The AE profile in patients aged >65 years was broadly consistent with that in patients aged ≤65 years (Table 4), except for a higher rate of grade ≥3 pneumonia in the older patients.
Discussion
This study demonstrates that pomalidomide in combination with low-dose dexamethasone is an effective regimen that confers disease stabilization or regression in 86 % of heavily pretreated Japanese patients with RRMM, with an acceptable safety profile consistent with the prior studies in other regions. The phase 3 MM-003 trial, which was conducted in 93 centers in Europe, Russia, Australia, Canada, and the United States, previously investigated pomalidomide plus dexamethasone in 302 patients with RRMM who had received prior therapy with both lenalidomide and bortezomib [9]. MM-003 found that pomalidomide plus low-dose dexamethasone significantly improved PFS, OS, and ORR vs high-dose dexamethasone alone [9].
Due to the small sample size of MM-011, results may be less precise and the ability to compare with other trials such as MM-003 is limited, therefore findings in our study should be interpreted with caution. However, the results reported here, including an ORR of 42 % (compared with 31 % in MM-003), suggest that efficacy outcomes of pomalidomide-based salvage treatment in RRMM could be more favorable depending on the criteria used for patient selection [9]. It will be interesting to determine if, as in MM-003, patients with a greater response (either SD or ≥PR) experience a longer OS compared with patients with progressive disease [16]. Additionally, median PFS in MM-011 was substantially longer than in the MM-003 trial (10.2 vs 4.0 months, respectively) [9], probably reflecting the differences in background characteristics of the patients participating in these studies.
One possible explanation for the observed differences in outcomes between MM-011 and MM-003 is the longer duration of treatment in MM-011 (median, 5.5 vs 4.2 months) [9]. Subgroup analysis of MM-003 showed that some patients who achieved only SD within four cycles of treatment went on to improve their response status with continued treatment beyond four cycles [16]. Thus, the improved outcomes in MM-011 may reflect prolonged time on therapy.
Additionally, the observed differences in outcomes may reflect variability in disease characteristics between the patient population of MM-011 and the pomalidomide plus low-dose dexamethasone arm of MM-003. Several factors associated with poor outcomes were less common in MM-011 than in MM-003. For example, impaired renal function (creatinine clearance <60 mL/min) was present in 22 % of patients in MM-011 compared with 31 % of patients in MM-003 [9]. Renal function may reflect the disease status of MM; however, preliminary data from the MM-008 study showed that pomalidomide dosing need not be reduced in patients with renal function impairment [17]. Additionally, slightly fewer patients in MM-011 had bone lesions compared with MM-003 (61 vs 68 %) [9]. Advanced lytic lesions were reported to be a risk factor associated with poor survival in patients who receive pomalidomide [18]. Finally, patients had lower levels of serum β2-microglobulin in MM-011 vs MM-003 (Celgene Corporation, MM-003 clinical study report, unpublished observation). Higher serum β2-microgloblin has been identified as a risk factor associated with shorter OS in patients refractory to bortezomib and IMiD immunomodulatory agents [2].
Of note, three out of five patients with plasmacytomas in MM-011 achieved SD following treatment with pomalidomide plus low-dose dexamethasone, albeit with a short median PFS of 1.8 months. Extramedullary disease associated with MM is known for poor prognosis, even after the introduction of novel agents, including lenalidomide and bortezomib, and thalidomide [19, 20]. Therefore, improved treatment options are urgently needed for this patient population. Because pomalidomide has been shown to have more potent in vitro antimyeloma activity compared with conventional IMiD agents [21–23], further studies are needed to determine whether pomalidomide plus low-dose dexamethasone provides a survival benefit in patients with extramedullary disease or nonsolitary plasmacytoma.
Use of prior treatment options also notably differed between MM-011 and MM-003. Fewer patients in MM-011 had received a prior stem cell transplant than patients in MM-003 (53 vs 71 %) [9]. Although patients had received a higher number of prior antimyeloma therapies in MM-011 vs MM-003 (median, 6.5 vs 5 therapies), median time from initial diagnosis was shorter in MM-011 (4.7 vs 5.3 years). A similar proportion of patients in MM-011 and MM-003 were refractory to lenalidomide (97.2 vs 95 %). Fewer patients in MM-011 were refractory to bortezomib alone (58 vs 79 %) or to both lenalidomide and bortezomib (58 vs 75 %). The lower levels of bortezomib refractory disease in MM-011 may result from differences in eligibility criteria: MM-003, but not MM-011, included patients with primary refractory disease and required that patients had experienced prior treatment failure with both lenalidomide and bortezomib. These differences suggest that patients in MM-011 had disease that was not advanced as in MM-003, potentially accounting for the higher response rates and longer PFS observed. However, subanalysis of MM-003 found no effect of prior treatment on response rate [16].
The chromosomal aberrations del(17p) and t(4;14) are associated with adverse prognosis, with median event-free survival from diagnosis of only 20.6 months and 15 months, respectively [24–27]. Therefore, the efficacy of pomalidomide plus low-dose dexamethasone in MM-011 may have been affected by the proportion of patients with these poor-risk chromosomal aberrations. However, collection of data on chromosomal aberrations was not included in the MM-011 protocol, and this analysis is not available.
The non-hematologic AE profile in MM-011 was generally consistent with that of pomalidomide plus low-dose dexamethasone treatment in MM-003, with a few exceptions. The incidence of severe infections was lower in MM-011 (8.3 % with grade ≥3 infection or infestation) than in MM-003 (30 % with grade 3/4 infection), as was the incidence of any grade febrile neutropenia (3 vs 10 %) [9]. Venous thromboembolism (VTE) is associated with decreased survival in MM [28] and is a rare but potentially serious AE that has been reported with IMiD therapy [29, 30]. In a preliminary study in 1035 Japanese patients with MM treated with thalidomide, the incidence of VTE was found to be lower than that in Western patients [31], potentially associated with genetic background and other factors related to ethnicity [32, 33]. In prior studies, appropriate thromboprophylaxis has been selected based on the risk of VTE for Japanese patients. With appropriate protocol-mandated thromboprophylaxis in MM-011, no cases of VTE were reported. Finally, PN is a common and potentially treatment-limiting AE associated with thalidomide and bortezomib; however, pomalidomide as well as lenalidomide do not appear to cause substantial neurotoxicity [9, 34]. In MM-011, PN of any grade occurred in three patients (8 %) and did not lead to treatment discontinuation.
Grade ≥3 hematologic AEs occurred more frequently in MM-011 than in MM-003, including neutropenia (64 vs 48 %), anemia (42 vs 33 %), and thrombocytopenia (31 vs 22 %); however, the rate of all-grade hematologic AEs in MM-011 was similar to MM-003 [9]. In the Japanese MM-004 study, the incidence of grade ≥3 neutropenia was also higher than in MM-003 (67 vs 48 %) [9, 13]. This suggests that a greater number of Japanese patients may have a greater need for dose adjustments in response to hematologic AEs compared with those from other regions. The observed differences in AEs are not likely to be due to PK differences, as MM-004 found PK parameters of pomalidomide in Japanese patients with RRMM to be similar to those reported for pomalidomide in other RRMM populations, with limited accumulation after multiple doses [13]. Importantly, hematologic AEs were manageable with temporary discontinuation of treatment or with concomitant administration of granulocyte colony-stimulating factor. The successful management of AEs may have contributed to extended duration of treatment in MM-011.
Conclusions
In conclusion, pomalidomide 4 mg/day has been confirmed as the acceptable starting dose for Japanese patients, with dexamethasone administered at a dose of 40 mg/day (reduced to 20 mg/day for patients aged >75 years). Pomalidomide plus low-dose dexamethasone is a relatively safe and highly efficacious treatment for Japanese patients with RRMM who have previously received both lenalidomide and bortezomib. Patients who achieve stable disease or better response while on pomalidomide can continue to benefit from this therapy. Additional studies may be required to further define those patients that would derive the most benefit from pomalidomide-based therapies.
Methods
Patients
Eligible patients had documented MM and relapsed and refractory disease, defined as disease progression after ≥SD for ≥1 cycle of treatment or during or within 60 days of completing treatment. Other inclusion criteria were ≥2 prior therapies (including ≥2 cycles of lenalidomide and bortezomib, separately or in combination), age ≥20 years, and Eastern Cooperative Oncology Group performance status ≤2. Exclusion criteria included previous pomalidomide treatment; hypersensitivity to thalidomide, lenalidomide, or dexamethasone; absolute neutrophil count <1000/μL; platelet count <75,000/μL (or <30,000/μL if ≥50 % of bone marrow nucleated cells were plasma cells); creatinine clearance <45 mL/min using the Cockcroft-Gault formula; corrected serum calcium >14 mg/dL (>3.5 mmol/L); hemoglobin <8 g/dL (<4.9 mmol/L); liver enzyme concentrations >3.0× upper limit of normal (ULN); total bilirubin >2.0 mg/dL (34.2 μmol/L; or ≥3.0× ULN for hereditary benign hyperbilirubinemia); congestive heart failure (New York Heart Association Class III/IV); myocardial infarction within 12 months; unstable or poorly controlled angina pectoris; and PN grade ≥2.
All patients provided informed consent; the study was approved by each study site’s institutional review board and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines on good clinical practice. The trial is registered as clinicaltrials.gov identifier NCT02011113.
Study design
MM-011 was a phase 2 multicenter, single-arm, open-label study conducted in Japan (Fig. 1). Patients received pomalidomide (4 mg/day orally, days 1-21, 28-day cycles) and dexamethasone (40 mg/day [20 mg/day if aged >75 years] orally, days 1, 8, 15, and 22), consistent with United States and European Union approved dosing [4, 5]. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal. All patients received thromboprophylaxis with low-dose aspirin, low-molecular-weight heparin, or equivalent.
Pomalidomide was interrupted for grade 4 neutropenia or thrombocytopenia, grade ≥3 constipation, VTE, rash, PN, or other pomalidomide-related AE, or grade ≥2 hypothyroidism or hyperthyroidism. Additionally, pomalidomide was interrupted for febrile neutropenia (any grade). Pomalidomide could be restarted at the same level or decreased by 1 mg. Discontinuation of pomalidomide was indicated for rash (grade 4 or blistering) or grade ≥4 PN. Dexamethasone dose was modified for grade ≥3 edema, hyperglycemia, or any other dexamethasone-related AE. Additionally, dexamethasone was modified for grade ≥2 confusion/mood alteration or muscle weakness, or any grade dyspepsia. Dexamethasone was discontinued for acute pancreatitis.
The primary endpoint was response rate according to the International Myeloma Working Group (IMWG) criteria [35]. Enrollment of 37 patients was planned using the expected response rate of 25 % based on the efficacy evaluable population, the threshold response rate of 10 %, on one-sided alpha of 0.05 and the statistical power of 80 % based on the test for one sample proportion. Secondary endpoints included response rate according to European Group for Blood and Marrow Transplantation (EBMT) criteria [36], time to response (TTR), DOR, PFS, and safety.
Efficacy assessments
Response was assessed by investigators using IMWG criteria and was confirmed by the members of an independent response adjudication committee, who also confirmed responses using EBMT criteria. TTR was calculated as the time from first dose to first documented response. DOR was defined as the time from first documented response to first documented disease progression. PFS was the time from first dose to first documented disease progression or death, whichever occurred earlier, and was estimated using the Kaplan–Meier method.
Safety evaluation
AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 [37] throughout treatment and for 28 days after last dose. Other safety assessments included VTE monitoring, physical examinations, vital signs, electrocardiograms, and standard clinical laboratory assessments (thyroid function, hematology, serum chemistry, urinalysis, creatinine clearance, and virology).
Abbreviations
- AE:
-
adverse event
- CR:
-
complete response
- DOR:
-
duration of response
- EBMT:
-
European Group for Blood and Marrow Transplantation
- ECOG:
-
Eastern Cooperative Oncology Group
- IMWG:
-
International Myeloma Working Group
- ITT:
-
intent to treat
- MM:
-
multiple myeloma
- ORR:
-
overall response rate
- OS:
-
overall survival
- PFS:
-
progression-free survival
- PK:
-
pharmacokinetics
- PN:
-
peripheral neuropathy
- PR:
-
partial response
- RRMM:
-
relapsed and refractory MM
- SD:
-
stable disease
- TTR:
-
time to response
- ULN:
-
upper limit of normal
- VGPR:
-
very good partial response
- VTE:
-
venous thromboembolism
References
Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20.
Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26:149–57 (Erratum in: Leukemia. 2012;26(5):1153).
Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDs) in multiple myeloma. Leukemia. 2010;24:22–32.
AnonymousImnovid (pomalidomide) [Summary of Product Characteristics]. Uxbridge, UK:Celgene Europe; 2015.
AnonymousPomalyst (pomalidomide) [package insert]. Summit, NJ:Celgene Corporation; 2015.
AnonymousPomalyst (pomalidomide) [product monograph]. Mississauga, ON:Celgene Canada; 2015.
Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–7.
Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–32.
San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–66.
Johnson JA. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther. 2000;38:53–60.
Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–23.
Ramamoorthy A, Pacanowski MA, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: review of recently approved drugs. Clin Pharmacol Ther. 2015;97:263–73.
Matsue K, Iwasaki H, Chou T, et al. Pomalidomide alone or in combination with dexamethasone in Japanese patients with refractory or relapsed and refractory multiple myeloma. Cancer Sci. 2015;106:1561–7.
Guglielmelli T, Petrucci MT, Saglio G, Palumbo A. Thalidomide after lenalidomide: a possible treatment regimen in relapse refractory multiple myeloma patients. Br J Haematol. 2011;153:146.
Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–66.
San Miguel JF, Weisel KC, Song KW, et al. Impact of prior treatment and depth of response on survival in MM-003, a randomized phase 3 study comparing pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone in relapsed/refractory multiple myeloma. Haematologica. 2015;100:1334–9.
Matous J, Siegel D, Lonial S, Harvey R, Kasserra C. MM-008: a phase 1 trial evaluating pharmacokinetics and tolerability of pomalidomide + low-dose dexamethasone in patients with relapsed or refractory and refractory multiple myeloma and renal impairment. Blood. 2014;124:4730.
Wang TF, Ahluwalia R, Fiala MA, et al. The characteristics and outcomes of patients with multiple myeloma dual refractory or intolerant to bortezomib and lenalidomide in the era of carfilzomib and pomalidomide. Leuk Lymphoma. 2014;55:337–41.
Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010;21:325–30.
Pour L, Sevcikova S, Greslikova H, et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relpase. Haematologica. 2014;99:360–4.
Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–6.
Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–6.
Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the anti-myeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–9.
Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–95.
Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome experience. J Clin Oncol. 2013;31:2806–9.
Leleu X, Karlin L, Macro M, et al. Pomalidomide plus low-dose dexamethasone in multiple myeloma with deletion 17p and/or translocation (4;14): IFM 2010-02 trial results. Blood. 2015;125:1411–7.
Dimopoulos MA, Weisel KC, Song KW, et al. Cytogenetics and long-term survival of pomalidomide and low-dose dexamethasone in refractory or relapsed and refractory multiple myeloma. Haematologica. 2015;100:1327–33.
Kristinsson SY, Pfeiffer RM, Bjorkholm M, Schulman S, Landgren O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica. 2012;97:1603–7.
Leleu X, Rodon P, Hulin C, et al. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thromb Haemost. 2013;110:844–51.
Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23.
Kato A, Takano H, Ichikawa A, et al. A retrospective cohort study of venous thromboembolism (VTE) in 1035 Japanese myeloma patients treated with thalidomide; lower incidence without statistically significant association between specific risk factors and development of VTE and effects of thromboprophylaxis with aspirin and warfarin. Thromb Res. 2013;131:140–4.
Roberts LN, Patel RK, Arya R. Venous thromboembolism and ethnicity. Br J Haematol. 2009;146:369–83.
Montagnana M, Favaloro EJ, Franchini M, Guidi GC, Lippi G. The role of ethnicity, age and gender in venous thromboembolism. J Thromb Thrombolysis. 2010;29:489–96.
Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol. 2010;11:1086–95.
Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; 2010.
Authors’ contributions
All authors were involved with data acquisition, interpretation of the data, review and revision of the manuscript for important intellectual content, and giving final approval of the version to be submitted. In addition, TI, YK, and SH were involved in the drafting of the manuscript, MZ, TD, CU, and HT contributed to the conception and design of the study, and analyzed the data. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank all the physicians and medical staff who dedicated their time for the care of patients who participated in this study. The authors also thank all the research coordinators and data managers who contributed to the study. The authors received editorial assistance from MediTech Media (Peter J. Simon, Ph.D and Roy Garcia, Ph.D), sponsored by Celgene Corporation. This study was supported by Celgene K.K.
Competing interests
TI received honoraria (Pfizer, Celgene) and research funding (Toyama Chemical, Kyowa Hakko Kirin, Pfizer, Chugai); SO and KM received honoraria (Celgene). SI received honoraria (Celgene, Janssen, Ono, Takeda, Bristol-Myers Squibb) and research funding (Celgene, Kyowa Hakko Kirin, Ono, Eli Lilly, Janssen, Bayer, Chugai, Takeda). KS received research funding (Takeda, Ono, Novartis, Celgene). KA received research funding (Kyowa Hakko Kirin). KT received honoraria (Zenyaku, Eisai, Spectrum, Takeda) and research funding (Chugai, Kyowa Hakko Kirin, Ono, Celgene, Janssen, GSK, Eisai, Mundipharma, Takeda, Servier, AbbVie). TC received honoraria and consultancy fee (Celgene, Janssen, Novartis, Ono). HK received research funding (Celgene). HI received honoraria (Celgene, Kyowa Hakko Kirin) and research funding (Kyowa Hakko Kirin, Bristol-Myers Squibb, Novartis). SH is a member of an advisory committee (Celgene). CU, HT, MZ and TD are employees of Celgene. The other authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tatsuo Ichinohe and Yoshiaki Kuroda co-first authors contributed equally to this work
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ichinohe, T., Kuroda, Y., Okamoto, S. et al. A multicenter phase 2 study of pomalidomide plus dexamethasone in patients with relapsed and refractory multiple myeloma: the Japanese MM-011 trial. Exp Hematol Oncol 5, 11 (2015). https://doi.org/10.1186/s40164-016-0040-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-016-0040-7