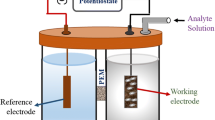
Abstract
Malic acid is an important fruit ripening indicator. Fruit industry losses every year due to non-availability of rapid technology for early detection of ripening of fruits. Therefore, nanosensor was developed for detection of malic acid concentrations in tomato at early stage of ripening before transport to the market. The enzyme NADP-malate dehydrogenase (Malic enzyme) was covalently immobilized on to screen printed carboxylated-multiwall carbon nanotubes working electrode using EDC-NHS chemistry. The enzyme electrode was characterized using scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) spectroscopy. The immobilized enzyme/c-MWCNT electrode was used for amperometric determination of different concentrations of malic acid in tomato using differential pulse voltammogram (DPV) at scan rate of 100 mv/s. The limit of detection of malic acid was 0.01 mM. The nanosensor showed low Km (0.12 mM), less response time (2 min), high sensitivity (0.01 mM) and better storage stability 180 days at 4 °C compared to earlier reported malate biosensor. The nanosensor was also validated at different stages of ripening of tomato using enzymatic method.

Nanosensor for detection of malic acid in tomato






Similar content being viewed by others
Change history
03 January 2018
The original version of this article unfortunately contained mistakes. In Figs. 2a, c and 5a in which Y-axis (Current X103) should not be written. It should only be “Current”. The correct version of Figs. 2a, c and 5a are given below.
References
Arif M, Setford SJ, Burton KS, Tothill IE (2002) L-malic acid biosensor for field based evaluation of apple, potato and tomato horticultural produce. Analyst 127:104–108
Arvinte A, Rotariu L, Bala C (2008) Amperometric low-potential detection of malic acid using single-wall carbon nanotubes based electrodes. Sensors 8:1497–1507
Barden TJ, Croft MY, Murby EJ, Wells RJ (1997) Gas chromatographic determination of organic acids from fruit juices by combined resin mediated methylation and extraction in supercritical carbon dioxide. J Chromatogr 785:251–261
Cannata JJB, Frasch ACC, Cataldi MA, Segura EL, Cazzulo JJ (1979) Two forms of malic enzyme with different regulatory properties in Trypanosoma cruzi. Biochemist 184:409–419
Davis JN (1966) Changes in the non-volatile organic acids of tomato fruit during ripening. J Sci Food Agri 17:396–400
Doubnerova V, Ryslava H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress. Plant Sci 180:575–583
Esti M, Volpe G, Micheli L, Delibato E, Compagnone D, Moscone D, Palleschi G (2004) Electrochemical biosensors for monitoring malolactic fermentation in red wine using two strains of Oenococcus oeni. Anal Chim Acta 513:357–364
Gajovic N, Warsinke A, Scheller FW (1997) Comparison of two enzyme sequences for a novel L-malate biosensor. J Chem Technol Biotechnol 68:31–36
Gautam P, Suniti S, Amrita PK, Madathil D, Nair BAN (2012) A review on recent advances in biosensors for detection of water contamination. Int J Env Sci 2:1565–1574
Goodenough PW, Prosser IM, Young K (1985) NADP-linked malic enzyme and malate metabolism in ageing tomato fruit. Phytochemistry 24:1157–1162
Gooding JJ, Erokhin P, Hibbert DB (2000) Parameters important in tuning the response of monolayer enzyme electrodes fabricated using self assembled monolayers of alkanethiols. Biosens Bioelectron 15:229–239
Harris PJF (2004) Carbon nanotube composites. Int Mater Rev 49:31–43
Hegde RN, Chandra P, Nandibewoor ST (2011) Sensitive voltammetric determination of atenolol at multi-walled carbon nanotubes modified glassy carbon electrode. J Nanosci Nanotechnol 1:75–86
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Jayapraksha GK, Sakariah KK (1998) Determination of organic acids in Garcinia cambogia. J Chromatogr 806:337–339
Kader AA (1999) Fruit maturity, ripening and quality relationships. Acta Hort 485:203–208
Kong J, Yu S (2007) Fourier transforms infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39:549–559
Kovacs E, Djedjro GA (1994) Changes in organic acids of fruits after different treatments. Acta Hort 368:219–226
Lin Y, Lu F, Tu Y, Ren Z (2004) Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett 4:191–195
Lupu A, Compagnone D, Palleschi G (2004) Screen-printed enzyme electrodes for the detection of marker analytes during winemaking. Anal Chim Acta 513:67–72
Maines A, Prodromidis MI, Tzouwara SM, Karayannis MI, Ashworth D, Vadgama P (2000) Reagentless enzyme electrode for malate based on modified polymeric membranes. Anal Chim Acta 408:217–224
Nugent JM, Santhanam KSV, Rubio A, Ajayan PM (2001) Fast electron transfer kinetics on multiwalled carbon nanotube microbundle electrode. Nano Lett 1:87–91
Prakash PA, Yogeswaran U, Chen SM (2009) A review on direct electrochemistry of catalase for electrochemical sensors. Sensors 9:1821–1844
Ruhal A, Rana JS, Kumar S, Kumar A (2012) Immobilization of malate dehydrogenase on carbon nanotubes for development of malate biosensor. Cell Mol Biol 58:15–20
Sharma AK, Vatsyayan P, Goswami P, Minteer SD (2009) Recent advances in material science for developing enzyme electrodes. Biosens Bioelectron 24(8):2313–2322
Siebert G, Ritter H, Kompf J (1979) Mitochondrial malic enzyme in human leukocytes: formal genetics and population genetics. Hum Genet 51:319–322
Sotiropoulou S, Chaniotakis NA (2003) Carbon nanotube array-based biosensor. Anal Bioanal Chem 375:103–105
Suye S, Yoshihara N, Inuta S (1992) Spectrophotometric determination of L-malic acid with a malic enzyme. Biosci Biotechnol Biochem 56:1488–1489
Tu Y, Lin Y, Yantasee W, Ren Z (2005) Carbon nanotubes based nanoelectrode arrays: fabrication, evaluation and application in voltammetric analysis. Electroanalysis 17:79–84
Zhang M, Smith A, Gorski W (2004) Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem 76(17):5045–5050
Acknowledgements
Anita Dalal thanks the State Government of Haryana for providing C.V. Raman fellowship to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anita Dalal declares that she has no conflict of interest. J. S. Rana declares that he has no conflict of interest. Ashok Kumar declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s12161-017-1119-4.
Rights and permissions
About this article
Cite this article
Dalal, A., Rana, J.S. & Kumar, A. Ultrasensitive Nanosensor for Detection of Malic Acid in Tomato as Fruit Ripening Indicator. Food Anal. Methods 10, 3680–3686 (2017). https://doi.org/10.1007/s12161-017-0919-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-017-0919-x