Abstract
Objective
2-[18F]fluoro-2-deoxy-d-glucose (FDG) is known to accumulate in benign conditions such as infection and inflammation as well as in malignancy. Vaccination may cause transient inflammation of lymph nodes, which may induce false-positive findings on FDG-positron emission tomography (PET) imaging. This study investigated the influence of influenza vaccination on FDG-PET/CT imaging in normal subjects.
Methods
Between November 2008 and March 2009, a total of 172 examinees underwent FDG-PET/CT during an annual cancer-screening program at our hospital, 83 of whom had a history of recent non-adjuvanted seasonal influenza vaccination. They were asked the date and injection site of the vaccination. Examinees were divided into 2 groups based on the interval after vaccination using a cutoff value of 7 days (1 week). Two double board-certified nuclear medicine physicians and radiologists visually interpreted the FDG-PET/CT images with reference to PET/CT fusion and CT images and checked the location and the number of abnormal accumulations by consensus reading.
Results
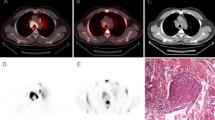
Intervals between vaccination and FDG-PET were less than 7 days in 5 examinees, and 7 days or more in 78 examinees. Unexpected accumulations were visualized in 4 examinees in the axilla and medial upper arm, and all of them belonged to the group who underwent vaccination less than 7 days previously. In the second group there was no abnormal FDG accumulation.
Conclusions
Recent influenza vaccination before FDG-PET/CT examination may cause ipsilateral axillary lymph node accumulations, especially within several days after vaccination. Questionnaires about vaccination can help to avoid false interpretation of FDG avid axillary lymph nodes.


Similar content being viewed by others
References
Stumpe KD, Dazzi H, Schaffner A, von Schulthess GK. Infection imaging using whole-body FDG-PET. Eur J Nucl Med. 2000;27:822–32.
Williams G, Joyce RM, Parker JA. False-positive axillary lymph node on FDG-PET/CT scan resulting from immunization. Clin Nucl Med. 2006;31:731–2.
Iyengar S, Chin B, Margolick JB, Sabundayo BP, Schwartz DH. Anatomical loci of HIV-associated immune activation and association with viraemia. Lancet. 2003;362:945–50.
Panagiotidis E, Exarhos D, Housianakou I, Bournazos A, Datseris I. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol. 2010;20:1251–3.
Thomassen A, Lerberg Nielsen A, Gerke O, Johansen A, Petersen H. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–8.
Burger IA, Husmann L, Hany TF, Schmid DT, Schaefer NG. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36:848–53.
Focosi D, Caracciolo F, Galimberti S, Papineschi F, Petrini M. False positive PET scanning caused by inactivated influenza virus vaccination during complete remission from anaplastic T-cell lymphoma. Ann Hematol. 2008;87:343–4.
Brown LE. The role of adjuvants in vaccines for seasonal and pandemic influenza. Vaccine. 2010;28:8043–5.
Minamimoto R, Senda M, Terauchi T, Jinnouchi S, Inoue T, Iinuma T, et al. Analysis of various malignant neoplasms detected by FDG-PET cancer screening program: based on a Japanese Nationwide Survey. Ann Nucl Med. 2011;25:45–54.
Minamimoto R, Senda M, Uno K, Jinnouchi S, Iinuma T, Ito K, et al. Performance profile of FDG-PET and PET/CT for cancer screening on the basis of a Japanese Nationwide Survey. Ann Nucl Med. 2007;21:481–98.
Barrington SF, O’Doherty MJ. Limitations of PET for imaging lymphoma. Eur J Nucl Med Mol Imaging. 2003;30:S117–27.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas, NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–53.
Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504–16.
Shien T, Akashi-Tanaka S, Yoshida M, Hojo T, Iwamoto E, Miyakawa K, et al. Evaluation of axillary status in patients with breast cancer using thin-section CT. Int J Clin Oncol. 2008;13:314–9.
Hara M, Tanaka K, Hirota Y. Immune response to influenza vaccine in healthy adults and the elderly: association with nutritional status. Vaccine. 2005;23:1457–63.
Sagawa M, Kojimahara N, Otsuka N, Kimura M, Yamaguchi N. Immune response to influenza vaccine in the elderly: association with nutritional and physical status. Geriatr Gerontol Int. 2011;11:63–8.
Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28:4123–9.
Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirone, N., Shinkai, T., Yamane, T. et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med 26, 248–252 (2012). https://doi.org/10.1007/s12149-011-0568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-011-0568-x