Abstract
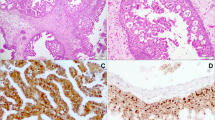
The role of human papillomavirus (HPV) as an etiologic and transformational agent in inverted Schneiderian papilloma (ISP) is unclear. Indeed, reported detection rates of HPV in ISPs range from 0 to 100%. The true incidence has been confounded by a tendency to conflate high- and low-risk HPV types and by the inability to discern biologically relevant from irrelevant HPV infections. The recent development of RNA in situ hybridization for high-risk HPV E6/E7 mRNA now allows the direct visualization of transcriptionally active high-risk HPV in ISP, providing an opportunity to more definitively assess its role in the development and progression of ISPs. We performed p16 immunohistochemistry and high-risk HPV RNA in situ hybridization on 30 benign ISPs, 7 ISPs with dysplasia, 16 ISPs with carcinomatous transformation, and 7 non-keratinizing squamous cell carcinomas (SCCs) with inverted growth that were unassociated with ISP. Transcriptionally active HPV was not detected in any of the 52 ISPs including those that had undergone carcinomatous transformation, but it was detected in two of seven (29%) non-keratinizing SCCs that showed inverted growth. There was a strong correlation between high-risk HPV RNA in situ hybridization and p16 immunohistochemistry (97%; p < 0.01). These results indicate that transcriptionally active high-risk HPV does not play a common role in either the development of ISP or in its transformation into carcinoma.



Similar content being viewed by others
References
Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–97.
Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206.
Lawson W, Schlecht NF, Brandwein-Gensler M. The role of the human papillomavirus in the pathogenesis of Schneiderian inverted papillomas: an analytic overview of the evidence. Head Neck Pathol. 2008;2:49–59.
Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic schneiderian papillomas. Laryngoscope. 2002;112:1372–7.
NORRIS HJ. Papillary lesions of the nasal cavity and paranasal sinuses. II. Inverting papillomas. A study of 29 cases. Laryngoscope. 1963;73:1–17.
Ridolfi RL, Lieberman PH, Erlandson RA, et al. Schneiderian papillomas: a clinicopathologic study of 30 cases. Am J Surg Pathol. 1977;1:43–53.
Furuta Y, Shinohara T, Sano K, et al. Molecular pathologic study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope. 1991;101:79–85.
Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105:178–83.
Nudell J, Chiosea S, Thompson LD. Carcinoma ex-Schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–86.
Califano J, Koch W, Sidransky D, et al. Inverted sinonasal papilloma: a molecular genetic appraisal of its putative status as a precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–7.
Udager AM, Rolland DC, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:2600–6.
Syrjanen KJ. HPV infections in benign and malignant sinonasal lesions. J Clin Pathol. 2003;56:174–81.
Syrjänen K, Syrjänen S. Detection of human papillomavirus in sinonasal papillomas: systematic review and meta-analysis. Laryngoscope. 2013;123:181–92.
Shah AA, Evans MF, Adamson CS, et al. HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16(INK4a) immunoreactivity. Head Neck Pathol. 2010;4:106–12.
Scheel A, Lin GC, McHugh JB, et al. Human papillomavirus infection and biomarkers in sinonasal inverted papillomas: clinical significance and molecular mechanisms. Int Forum Allergy Rhinol. 2015;5:701–7.
Ogura H, Fukushima K, Watanabe S. A high prevalence of human papillomavirus DNA in recurrent nasal papillomas. J Med Microbiol. 1996;45:162–6.
McKay SP, Grégoire L, Lonardo F, et al. Human papillomavirus (HPV) transcripts in malignant inverted papilloma are from integrated HPV DNA. Laryngoscope. 2005;115:1428–31.
Lewis JS, Westra WH, Thompson LD, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8:241–9.
Justice JM, Davis KM, Saenz DA, et al. Evidence that human papillomavirus causes inverted papilloma is sparse. Int Forum Allergy Rhinol. 2014;4:995–1001.
Jenko K, Kocjan B, Zidar N, et al. In inverted papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Arch. 2011;459:529–38.
Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–92.
Beck JC, McClatchey KD, Lesperance MM, et al. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:49–55.
Weiner JS, Sherris D, Kasperbauer J, et al. Relationship of human papillomavirus to Schneiderian papillomas. Laryngoscope. 1999;109:21–6.
Weber RS, Shillitoe EJ, Robbins KT, et al. Prevalence of human papillomavirus in inverted nasal papillomas. Arch Otolaryngol Head Neck Surg. 1988;114:23–6.
Tang AC, Grignon DJ, MacRae DL. The association of human papillomavirus with Schneiderian papillomas: a DNA in situ hybridization study. J Otolaryngol. 1994;23:292–7.
McLachlin CM, Kandel RA, Colgan TJ, et al. Prevalence of human papillomavirus in sinonasal papillomas: a study using polymerase chain reaction and in situ hybridization. Mod Pathol. 1992;5:406–9.
Kraft M, Simmen D, Casas R, et al. Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol. 2001;115:709–14.
Kim JY, Yoon JK, Citardi MJ, et al. The prevalence of human papilloma virus infection in sinonasal inverted papilloma specimens classified by histological grade. Am J Rhinol. 2007;21:664–9.
Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, human papilloma virus infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol (R Coll Radiol). 2006;18:300–5.
Kashima HK, Kessis T, Hruban RH, et al. Human papillomavirus in sinonasal papillomas and squamous cell carcinoma. Laryngoscope. 1992;102:973–6.
Hoffmann M, Klose N, Gottschlich S, et al. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006;239:64–70.
Gaffey MJ, Frierson HF, Weiss LM, et al. Human papillomavirus and Epstein-Barr virus in sinonasal Schneiderian papillomas. An in situ hybridization and polymerase chain reaction study. Am J Clin Pathol. 1996;106:475–82
Cheung FM, Lau TW, Cheung LK, et al. Schneiderian papillomas and carcinomas: a retrospective study with special reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J. 2010;89:E5–12.
Buchwald C, Lindeberg H, Pedersen BL, et al. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–10.
Schwerer MJ, Sailer A, Kraft K, et al. Patterns of p21(waf1/cip1) expression in non-papillomatous nasal mucosa, endophytic sinonasal papillomas, and associated carcinomas. J Clin Pathol. 2001;54:871–6.
Ukpo OC, Flanagan JJ, Ma XJ, et al. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–50.
Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–9.
Schache AG, Liloglou T, Risk JM, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–9.
Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–82.
Kerr DA, Arora KS, Mahadevan KK, et al. Performance of a branch chain RNA in situ hybridization assay for the detection of high-risk human papillomavirus in head and neck squamous cell carcinoma. Am J Surg Pathol. 2015;39:1643–52.
Mirghani H, Casiraghi O, Amen F, et al. Diagnosis of HPV-driven head and neck cancer with a single test in routine clinical practice. Mod Pathol. 2015;28:1518–27.
Rooper LM, Gandhi M, Bishop JA, et al. RNA in-situ hybridization is a practical and effective method for determining HPV status of oropharyngeal squamous cell carcinoma including discordant cases that are p16 positive by immunohistochemistry but HPV negative by DNA in-situ hybridization. Oral Oncol. 2016;55:11–6.
Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–73.
Grønhøj Larsen C, Gyldenløve M, Jensen DH, et al. Correlation between human papillomavirus and p16 overexpression in oropharyngeal tumours: a systematic review. Br J Cancer. 2014;110:1587–94.
Ash JE, Beck MR, Wilkes JD. Tumors of the upper respiratory tract and ear. Washington, DC: Armed Forces Institute of Pathology; 1964.
Hyams VJ, Batsakis JG, Michaels L, et al. Tumors of the upper respiratory tract and ear. Washington, D.C.: Armed Forces Institute of Pathology : Supt. of Docs., U.S. G.P.O. For sale by the Armed Forces Institute of Pathology; 1988.
El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–72.
Funding
This study was funded by the National Institutes of Dental and Craniofacial Research (R01 DE013152-11).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rooper, L.M., Bishop, J.A. & Westra, W.H. Transcriptionally Active High-Risk Human Papillomavirus is Not a Common Etiologic Agent in the Malignant Transformation of Inverted Schneiderian Papillomas. Head and Neck Pathol 11, 346–353 (2017). https://doi.org/10.1007/s12105-017-0779-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-017-0779-0