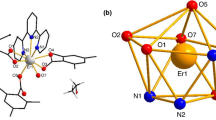
Abstract
A lanthanide carboxylates cluster derived from 1,8-naphthalene dicarboxylate (NDC= 1,8 naphthalene dicarboxylate) and 1,10-phenanthroline (Phen) has been synthesized. The cluster of Sm(III) has been utilized for luminescence and magnetic refrigeration properties. Most interestingly, the auto immobilization of atmospheric carbon dioxide forms carbonate ion which acts as a bridging ligand and is positioned at the middle of the cluster. This cluster is characterized by different spectroscopic tools like FT-IR, photoluminescence spectrum, and the molecular structure [Sm4(NDC)5(Phen)4(µ4-CO3)(H2O)3].3H2O.CH3OH (1) is determined by single crystals X-ray diffraction. π-conjugated ligand (NDC=1,8-Naphthalene dicarboxylate; Phen=phenanthroline) affects both absorption and photoluminescence intensity. Moreover, from the χmT vs temperature plot, it is observed that there is an occurrence of antiferromagnetic interaction among the SmIII centers. The cluster possesses high magnetocaloric value at low temperature which offers itself as a potential candidate for cryogenic molecular magnetic refrigerant material. In addition, thermogravimetric analysis, Hirsh field surface area analysis, and the optical diffuse reflectance spectrum of this cluster is also described.
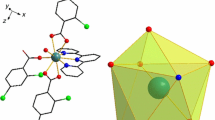
Graphical abstract
The synthesis, characterization, and magnetic properties of the Samarium cluster are described. The cluster is formed by the capturing of atmospheric CO2 in the form of carbonate ions which connects all four metal centers. The cluster exhibits a high magnetocaloric effect for which it can be used for molecular magnetic refrigerant material. Besides this, it can be a potential candidate for luminescent materials.









Similar content being viewed by others
References
Allen M R, Frame D J, Huntingford C, Jones C D, Lowe J A, Meinshausen M and Meinshausen N 2009 Warming caused by cumulative carbon emissions towards the trillionth tonne Nature 458 1163
Decortes A, Castilla A M and Kleij A W 2010 Salen-Complex-Mediated Formation of Cyclic Carbonates by Cycloaddition of CO2 to Epoxides Angew. Chem. Int. Ed. 49 9822
Langley S K, Moubaraki B and Murray K S 2012 Magnetic properties of hexanuclear lanthanide(III) clusters incorporating a central μ6-carbonate ligand derived from atmospheric CO2 fixation Inorg. Chem. 51 3947
He L, Nath J K and Lin Q 2019 Robust multivariate metal–porphyrin frameworks for efficient ambient fixation of CO2 to cyclic carbonates Chem. Commun. 55 412
Kitajima N, Fujisawa K, Koda T, Hikichi S and Moro-oka Y 1990 Fixation of atmospheric CO2 by a copper(II) complex J. Chem. Soc. Chem. Commun. 26 1357
Zhang B, Zheng X, Su H, Zhu Y, Du C and Song M 2013 Efficient fixation of atmospheric CO2 as carbonate by lanthanide-based complex via synergistic effect of zinc ion Dalton Trans. 42 8571
Maity S, Ghosh T K, Ito S, Bhunia P, Ishida T and Ghosh A 2022 Structures and Magnetic Properties of Carbonato-Bridged Hexanuclear NiII4LnIII2 (Ln = Gd, Tb, Dy) Complexes Formed by Atmospheric Carbon Dioxide Fixation in the Absence of an External Base Cryst. Growth Des. 22 4332
Bag P, Dutta S, Biswas P, Maji S K, Ulrich Flörke U and Nag K 2012 Fixation of carbon dioxide by macrocyclic lanthanide(III) complexes under neutral conditions producing self-assembled trimeric carbonato-bridged compounds with μ3-η2:η2:η2 bonding Dalton Trans. 41 3414
Tang X-L, Wang W-H, Dou W, Jiang J, Liu W-S, Qin W-W, et al. 2009 Olive-shaped chiral supramolecules: simultaneous self-assembly of heptameric lanthanum clusters and carbon dioxide fixation Angew. Chem. Int. Ed. 48 3499
Bian S-D, Jia J-H and Wang Q-M 2009 High-Nuclearity Silver Clusters Templated by Carbonates Generated from Atmospheric Carbon Dioxide Fixation J. Am. Chem. Soc. 113 3422
Tanase T, Nitta S, Yoshikawa S, Kobayashi K, Sakurai T and Yano S 1992 Spontaneous fixation of carbon dioxide in air by a nickel diamine complex: synthesis and characterization of a trinuclear nickel(II) complex with a novel hydrogen bonding system around a carbonate ligand Inorg. Chem. 31 1058
Zhang P, Zhang L, Lin S and –Y and Tang J 2013 Tetranuclear [MDy]2 Compounds and Their Dinuclear [MDy] (M = Zn/Cu) Building Units: Their Assembly, Structures, and Magnetic Properties Inorg. Chem. 52 6595
Titos-Padilla S, Ruiz J, Herrera J M, Brechin E K, Wersndorfer W, Lloret F and Colacio E 2013 Dilution-Triggered SMM Behavior under Zero Field in a Luminescent Zn2Dy2 Tetranuclear Complex Incorporating Carbonato-Bridging Ligands Derived from Atmospheric CO2 Fixation Inorg. Chem. 52 9620
Sakamoto S, Fujinami T, Nishi K, Matsumoto N, Mochida N, Ishida T, et al. 2013 Carbonato-Bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) Complexes Generated by Atmospheric CO2 Fixation and Their Single-Molecule-Magnet Behavior: [(μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn = N, N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato] Inorg. Chem. 52 7218
Ruiz J, Lorusso G, Evangelisti M, Brechin E K, Pope S J A and Colacio E 2014 Closely-Related ZnII2LnIII2 Complexes (LnIII = Gd, Yb) with Either Magnetic Refrigerant or Luminescent Single-Molecule Magnet Properties Inorg. Chem. 53 3586
Upadhyay A, Das C, Langley S K, Murray K S, Srivastava A K and Shanmugam M 2016 Heteronuclear Ni(ii)–Ln(iii) (Ln = La, Pr, Tb, Dy) complexes: synthesis and single-molecule magnet behaviour Dalton Trans. 45 3616
Liu C -M, Hao X and Zhang D-Q 2020 CO2-fixation into carbonate anions for the construction of 3d-4f cluster complexes with salen-type Schiff base ligands: from molecular magnetic refrigerants to luminescent single-molecule magnets Appl. Organomet Chem. 5893
Woodruff D N, Winpenny R E P and Layfield R A 2013 Lanthanide Single-Molecule Magnets Chem. Rev. 113 5110
Nath J K, Lan Y, Powell A K and Baruah J B 2013 Effect of Ancillary Ligands in Hydrolysis of 1,8-Naphthalic Anhydride for Synthesis of Metallacycles of Co2+, Ni2+, and Zn2+ Z. Anorg. Allg. Chem. 638 2250
Feng X, Guo N, Chen H P, Wang H, Yue L, Chen X, et al. 2017 Series anionic host coordination polymers based on azoxybenzene carboxylate: structures, luminescence, and magnetic properties Dalton Trans. 46 14192
Rubi K, Kumar P, Repaka D V M, Chen R, Wang J-S and Mahendirana R 2014 Giant magnetocaloric effect in magnetoelectric Eu1-xBaxTiO3 Appl. Phys. Lett. 104 032407
Terada N and Mamiya H 2021 High-efficiency magnetic refrigeration using holmium Nat. Commun. 12 1212
Han S-D, Li J-H, Liu H-H and Wang 2017 G -M 2017 Two hybrid lanthanide complexes exhibiting a large magnetocaloric effect and slow magnetic relaxation Dalton Trans. 46 10023
Das C, Upadhyay A, Ansari K U, Ogiwara N, Kitao T, Horike S and Shanmugam M 2018 Lanthanide-Based Porous Coordination Polymers: Syntheses, Slow Relaxation of Magnetization, and Magnetocaloric Effect Inorg. Chem. 57 6584
Kalita P, Goura J, Nayak P, Colacio E and Chandrasekhar V 2021 Octanuclear Ln8 complexes: magneto-caloric effect in the Gd8 analogue J. Chem. Sci. 133 82
Wang S Y, Wang W M, Zhang H X, Shen H Y, Jiang L, Cui J Z and Gao H L 2016 Seven phenoxido-bridged complexes encapsulated by 8-hydroxyquinoline Schiff base derivatives and β-diketone ligands: single-molecule magnet, magnetic refrigeration and luminescence properties Dalton Trans. 45 3362
Shen H-Y, Wang W-M, Bi Y-X, Gao H-L, Liu S and Cui J-Z 2015 Luminescence, magnetocaloric effect and single-molecule magnet behavior in lanthanide complexes based on a tridentate ligand derived from 8-hydroxyquinoline Dalton Trans. 44 18893
Cui C, Ju W W, Luo X M, Lin Q F, Cao J P and Xu Y 2018 A Series of Lanthanide Compounds Constructed from Ln8 Rings Exhibiting Large Magnetocaloric Effect and Interesting Luminescence Inorg. Chem. 57 8608
Biswas S, Das S, Leusen J V, Kögerler P and Chandrasekhar V 2015 Pentanuclear [2.2] spirocyclic lanthanide(iii) complexes: slow magnetic relaxation of the DyIII analogue Dalton Trans. 44 19282
Hooda P, Taxak V B, Malik R K, Khatri S, Kumari P, Khatkar S P and Kumar R 2022 Applicability of reddish-orange light emitting samarium (III) complexes for biomedical and multifunctional optoelectronic devices J. Fluores. 32 613
Li R-F, Li R-H, Liu X-F, Chang X-H and Feng X 2020 Lanthanide complexes based on a conjugated pyridine carboxylate ligand: structures, luminescence and magnetic properties RSC Adv. 10 6192
Thomas J and Ambili K S 2015 Synthesis, crystal structure and luminescent properties of a new samarium-fluorescein metal-organic framework J. Mol. Struct. 1098 167
Zong G-C, Huo J-X, Ren N, Zhang J-J, Qi X-X, Gao J, et al. 2015 Preparation, characterization, and properties of four new trivalent lanthanide complexes constructed using 2-bromine-5-methoxybenzoic acid and 1,10-phenanthroline Dalton Trans. 44 14877
Zheng Y X, Fu L S, Zhou Y H, Yu J B, Yu Y N, Wang S B and Zhang H J 2002 Electroluminescence based on a β-diketonate ternary samarium complex J. Mater. Chem. 12 919
Deng R, Yu J, Zhang H, Zhou L, Peng Z, Li Z and Guo Z 2007 Photoluminescence and electroluminescence properties of a samarium complex Sm(TTA)3phen Chem. Phys. Lett. 443 258
Bruker 2012 Smart Apex II (Bruker AXS Inc.: Madison, Wisconsin, USA)
Sheldrick G M 2015 SHELXT – Integrated space-group and crystal structure determination Acta Cryst. A71 3
Macrae C F, Sovago I, Cottrell S J, Galek P T A, McCabe P, Pidcock E, et al. 2020 Mercury 4.0: from visualization to analysis, design, and prediction J. Appl. Cryst. 53 226
Brandenburg K and Berndt M 1999 Diamond. Crystal Impact Gb R, Bonn, Germany
Turner M J, McKinnon J J, Wolff S K, Grimwood D J, Spackman P R, Jayatilaka D, Spackman M A 2017. Crystal Explorer 17, University of Western Australia
de Almeida L R, Carvalho J P S, Napolitano H B, Oliveira S S, Camargo A J, Figueredo A S, et al. 2017 Contribution of Directional Dihydrogen Interactions in the Supramolecular Assembly of Single Crystals: Quantum Chemical and Structural Investigation of C17H17N3O2 Azine Cryst. Growth Des. 17 5145
Prins L J, Reinhoudt D N and Timmerman P 2001 Noncovalent Synthesis Using Hydrogen Bonding Angew. Chemie Int. Ed. 40 2382
Liang X, Parkinson J A, Parsons S, Weishaupl M and Sadler P J 2002 Cadmium Cyclam Complexes: Interconversion of Cis and Trans Configurations and Fixation of CO2 Inorg. Chem. 41 4539
Wesley W M and Harry W G H 1966 Reflectance Spectroscopy (New York: Wiley) pp. 104−169
Pankove J I 1997 Optical Processes in Semiconductors (Englewood Cliffs, NJ: Prentice Hall) pp.34-86
Tang X, Sepehri-Amin H, Terada N et al. 2022 Magnetic refrigeration material operating at a full temperature range required for hydrogen liquefaction Nat. Commun. 13 1817
Kurti N, Robinson F N H, Simon F and Spohr D A 1956 Nuclear cooling Nature 178 450
Numazawa T, Kamiya K, Utaki T and Matsumoto K 2014 Magnetic refrigerator for hydrogen liquefaction Cryogenics 62 185
Rovenzano V, Shapiro A J and Shull R D 2004 Reduction of hysteresis losses in the magnetic refrigerant Gd5Ge2Si2 by the addition of iron Nature 429 853
Acknowledgements
The author acknowledged the central instrument facility, IIT Guwahati for instrument support, and S. B. Deorah College for the lab facility.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nath, J.K., Borah, R. A lanthanide cluster formed by fixing atmospheric CO2 to carbonate: a molecular magnetic refrigerant and photoluminescent material. J Chem Sci 135, 58 (2023). https://doi.org/10.1007/s12039-023-02176-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02176-z