Abstract
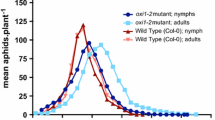
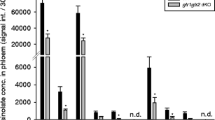
In Arabidopsis thaliana (Arabidopsis) treated with the harpin protein HrpNEa, resistance to the green peach aphid Myzus persicae, a generalist phloem-feeding insect, develops with induced expression of the AtMYB44 gene. Special GLUCAN SYNTHESIS-LIKE (GSL) genes and β-1,3-glucan callose play an important role in plant defence responses to attacks by phloem-feeding insects. Here we report that AtGLS5 and AtMYB44 are both required for HrpNEa-induced repression of M. persicae feeding from the phloem of Arabidopsis leaves. In 24 h successive surveys on large-scale aphid populations, the proportion of feeding aphids was much smaller in HrpNEa-treated plants than in control plants, and aphids preferred to feed from the 37 tested atgsl mutants rather than the wild-type plant. The atgsl mutants were generated previously by mutagenesis in 12 identified AtGSL genes (AtGSL1 through AtGSL12); in the 24 h survey, both atgsl5 and atgsl6 tolerated aphid feeding, and atgsl5 was the most tolerant. Consistently, atgsl5 was also most inhibitive to the deterrent effect of HrpNEa on the phloem-feeding activity of aphids as monitored by the electrical penetration graph technique. These results suggested an important role of the AtGSL5 gene in the effect of HrpNEa. In response to HrpNEa, AtGSL5 expression and callose deposition were induced in the wild-type plant but not in atgsl5. In response to HrpNEa, moreover, the AtMYB44 gene known to be required for repression of aphid reproduction on the plant was also required for repression of the phloem-feeding activity. Small amounts of the AtGSL5 transcript and callose deposition were detected in the atmyb44 mutant, as in atgsl5. Both mutants performed similarly in tolerating the phloem-feeding activity and impairing the deterrent effect of HrpNEa, suggesting that AtGSL5 and AtMYB44 both contributed to the effect.






Similar content being viewed by others
Abbreviations
- EPG:
-
electrical penetration graph
- GSL :
-
GLUCAN SYNTHESIS-LIKE
- HRB:
-
hexyl rhodamine B
- RT-PCR:
-
reverse transcriptase–polymerase chain reaction
- WT:
-
wild type
References
Alonso JM, Hirayama T, Roman G, Nourizadeh S and Ecker JR 1999 EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, et al. 2003 Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657
Boevink P, Cruz S, Hawes C, Harris N and Oparka KJ 1996 Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 10 935–941
Camehl I, Sherameti I, Venus Y, Bethke G, Varma A, Lee J and Oelmüller R 2010 Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 185 1062–1073
Chen H, Xue L, Chintamanani S, Germain H, Lin H, Cui H, Cai R, Zuo J, et al. 2009a ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21 2527–2540
Chen L, Qian J, Qu SP, Long JY, Yin Q, Zhang CL, Wu XJ, Sun F, et al. 2008 Identification of specific fragments of HpaGXooc, a harpin from Xanthomonas oryzae pv. oryzicola, that induce disease resistance and enhance growth in plants. Phytopathology 98 781–791
Chen XY, Liu L, Lee EK, Han X, Rim Y, Chu H, Kim SW, Sack F and Kim JY 2009b The Arabidopsis callose synthase gene, GSL8, is required for cytokinesis and cell patterning. Plant Physiol. 150 105–113
Chen XY and Kim JY 2009 Callose synthesis in higher plants. Plant Signal. Behav. 4 489–492
Consonni C, Bednarek P, Humphry M, Francocci F, Ferrari S, Harzen A, van Themaat EV and Panstruga R 2009 Tryptophan-derived metabolites are required for antifungal defence in the Arabidopsis thaliana mlo2 mutant. Plant Physiol. 152 1544–1561
De Vos M and Jander G 2009 Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 32 1548–1560
Dong HP, Peng JL, Bao ZL, Meng XD, Bonasera JM, Chen GY, Beer SV and Dong HS 2004 Downstream divergence of the ethylene signaling pathway for harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol. 136 3628–3638
Dong HP, Yu HQ, Bao ZL, Guo XJ, Peng JL, Yao Z, Chen GY and Dong HS 2005a The ABI2-dependent abscisic acid signalling controls HrpN-induced drought tolerance in Arabidopsis. Planta 221 313–327
Dong HS, Delaney TP, Bauer DW and Beer SV 1999 Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 20 207–215
Dong XY, Hong ZL, Chatterjee J, Kim S and Verma DP 2008 Expression of callose synthase genes and its connection with Npr1 signaling pathway during pathogen infection. Planta 229 87–98
Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M and Verma DP 2005b Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 42 315–328
Douglas AE 2006 Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57 747–754
Enns LC, Kanaoka MM, Torii KU, Comai L, Okada K and Cleland RE 2005 Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Mol. Biol. 58 333–349
Gou ZH, Zhang SP and Dong HS 2009 Effects of HrpNEa on inducing Aphid repellency on Cucumis melo. Acta Agric. Boreali-Sinica 24 188–192
Guo H and Ecker JR 2004 The ethylene signaling pathway: new insights. Curr. Opin. Plant Biol. 7 40–49
Hong Z, Delauney AJ and Verma DP 2001 A cell plate–specific callose synthase and its interaction with phragmoplastin. Plant Cell 13 755–768
Huang LJ, Chen XY, Rim Y, Han X, Cho WK, Kim SW and Kim JY 2008 Arabidopsis glucan synthase-like 10 functions in male gametogenesis. J. Plant Physiol. 166 344–352
Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P and Fincher GB 2003 An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15 2503–2513
Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD and Cheong JJ 2008 Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 146 623–635
Jung C, Shim JS, Seo JS, Lee HY, Kim CH, Choi YD and Cheong JJ 2010 Non-specific phytohormonal induction of AtMYB44 and suppression of jasmonate-responsive gene activation in Arabidopsis thaliana. Mol. Cell 29 71–76
Kaliff M, Staal J, Myrenås M and Dixelius C 2007 ABA is required for Leptosphaeria maculans resistance via ABI1- and ABI4-dependent signalling. Mol. Plant-Microbe Interact. 20 335–345
Kehr J 2006 Phloem sap proteins: their identities and potential roles in the interaction between plants and phloem-feeding insects. J. Exp. Bot. 57 767–774
Kim JF and Beer SV 2000 hrp genes and harpins of Erwinia amylovora: a decade of discovery; in Fire blight and its causative agent, Erwinia amylovora (ed.) JL Vanneste (Wallingford: CAB International) pp 141–162
Kirik V, Kolle K, Misera S and Baumlein H 1998 Two novel MYB homologues with changed expression in late embryogenesis-defective Arabidopsis mutants. Plant Mol. Biol. 37 819–827
Klingler J, Creasy R, Gao L, Nair RM, Calix AS, Jacob HS, Edwards OR and Singh KB 2005 Aphid resistance in Medicago trunculata involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 137 1445–1455
Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M and Martin C 1998 Towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J. 16 263–276
Kuśnierczyk A, Winge P, Jørstad TS, Troczyńska J, Rossiter JT and Bones AM 2008 Towards global understanding of plant defence against aphids – timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 31 1097–1115
Kwack MS, Eui Nam Kim EN, Lee H, Kim J-W, Chun S-C and Kim KD 2005 Digital image analysis to measure lesion area of cucumber anthracnose by Colletotrichum orbiculare. J. Gen. Plant Pathol. 71 418–421
Liu FQ, Liu HX, Jia Q, Wu XJ, Guo XJ, Zhang SJ, Song F and Dong HS 2006 The internal glycine-rich motif and cysteine suppress several effects of the HpaGXooc protein in plants. Phytopathology 96 1052–1059
Liu RX, LÜ BB, Wang XM, Zhang CL, Zhang SP, Qian J, Chen L, Shi HJ and Dong HS 2010a Thirty-seven transcription factor genes differentially respond to a harpin protein and affect resistance to the green peach aphid in Arabidopsis. J. Biosci. 35 435–450
Liu R, Chen L, Jia Z, Lü B, Shi H, Shao W and Dong H 2010b Transcription factor AtMYB44 regulates induced expression of the ETHYLENE INSENSITIVE2 gene in Arabidopsis responding to a harpin protein. Mol. Plant-Microbe Interact. 24 377–389
Louis J, Leung Q, Pegadaraju V, Reese J and Shah J 2010 PAD4-dependent antibiosis contributes to the ssi2-conferred hyper-resistance to the green peach aphid. Mol. Plant-Microbe Interact. 23 618–627
Mutti NS, Louis J, Pappan LK, Pappan K, Begum K, Chen M, Park Y, Dittmer N, Marshall J, Reese JC and Reeck GR 2008 A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad Sci. USA 105 9965–9969
Nishimura MT, Stein M, Hou B, Vogel JP, Edwards H and Somerville SC 2003 Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301 969–972
Pegadaraju V, Louis J, Singh V, Reese JC, Bautor J, Feys BJ, Cook G, Parker JE and Shah J 2007 Phloem-based resistance to green peach aphid is controlled by Arabidopsis PHYTOALEXIN DEFICIENT4 without its signaling partner ENHANCED DISEASE SUSCEPTIBILITY1. Plant J. 52 332–341
Peng JL, Bao ZL, Dong HS, Ren HY and Wang JS 2004 Expression of harpinXoo in transgenic tobacco induces pathogen defense in the absence of hypersensitive cell death. Phytopathology 94 1048–1055
Peng JL, Dong HS, Dong HP, Delaney TP, Bonasera BM and Beer SV 2003 Harpin-elicited hypersensitive cell death and pathogen resistance requires the NDR1 and EDS1 genes. Physiol. Mol. Plant Pathol. 62 317–326
Pitzschke A, Djamei A, Teige M and Hirt H 2009 VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc. Natl. Acad. Sci. USA 106 18414–18419
Pollard DG 1972 Plant penetration by feeding aphids (Hemiptera, Aphidoidea): A review. Bull. Entomol. Res. 62 631–714
Ren XY, Liu F, Bao ZL, Zhang CL, Wu XJ, Chen L, Liu RX and Dong HS 2008 Root growth of Arabidopsis thaliana is regulated by ethylene and abscisic acid signaling interaction in response to HrpNEa, a bacterial protein of harpin group. Plant Mol. Biol. Rep. 26 225–240
Reuber TL, Plotnikova JM, Dewdney J, Rogers EE, Wood W and Ausubel FM 1998 Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J. 16 473–485
Saheed SA, Cierlik I, Larsson KA, Delp G, Bradley G, Jonsson LM and Botha CE 2009 Stronger induction of callose deposition in barley by Russian wheat aphid than bird cherry-oat aphid is not associated with differences in callose synthase or β-1,3-glucanase transcript abundance. Physiol. Plant 135 150–161
Stone BA and Clarke AE 1992 Chemistry and physiology of higher plant 1,3-β-glucans (callose); in Chemistry and biology of 1,3-β-glucans (eds) BA Stone and AE Clarke (Bundoora Australia: La Trobe University Press) pp 365–429
Sun LJ, Ren HY, Liu RX, Li BY, Wu TQ, Sun F, Liu HM, Wang XM and Dong HS 2010 An h-type thioredoxin functions in tobacco defense responses to two species of viruses and an abiotic oxidative stress. Mol. Plant-Microbe Interact. 23 1470–1485
Tjallingii WF 1987 Electrical recording of stylet penetration activities; in Aphids: Their Biology, Natural Enemies and Control (eds) AK Minks and P Harrewijn (Elsevier Amsterdam) pp 95–108
Tjallingii WF 2006 Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 57 739–745
Tjallingii WF and Esch TH 1993 Fine-structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 18 317–328
Töller A, Brownfield L, Neu C, Twell D and Schulze-Lefert P 2008 Dual function of Arabidopsis Glucan Synthase-Like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J. 54 911–923
van der Zwet T and Beer SV 1999 Fire Blight–Its Nature, Prevention and Control: A Practical Guide to Integrated Disease Management. Agriculture Information Bulletin (U.S. Department of Agriculture) No. 631
Verma DPS and Hong Z 2001 Plant callose synthase complexes. Plant Mol. Biol. 47 693–701
Villada ES, González EG, López-Sesé AI, Castiel AF and Gómez-Guillamón ML 2009 Hypersensitive response to Aphis gossypii Glover in melon genotypes carrying the Vat gene. J. Exp. Bot. 60 3269–3277
Wang KL, Li H and Ecker JR 2002 Ethylene biosynthesis and signaling networks. Plant Cell 14 S131–151
Wawrzynska A, Rodibaugh NL and Innes RW 2010 Synergistic activation of defense responses in Arabidopsis by simultaneous loss of the GSL5 callose synthase and the EDR1 protein kinase. Mol. Plant-Microbe Interact. 23 578–584
Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A and Beer SV 1992 Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257 85–88
Will T and van Bel AJE 2006 Physical and chemical interactions between aphids and plants. J. Exp. Bot. 57 729–737
Will T and van Bel AJ 2008 Induction as well as suppression: How aphid saliva may exert opposite effects on plant defense. Plant Signal. Behav. 3 427–430
Willats WGT and Knox JP 2003 Molecules in context: probes for cell wall analysis; in The Plant Cell Wall (ed.) JKC Rose (Oxford UK: CRC) pp 92–110
Xie B, Wang X and Hong Z 2009 Precocious pollen germination in Arabidopsis plants with altered callose deposition during microsporogenesis. Planta 231 809–823
Zhang CL, Bao Z, Liang Y, Yang X, Wu XJ, Hong XY and Dong HS 2007 Abscisic acid mediates Arabidopsis drought tolerance induced by HrpNEa in the absence of ethylene signalling. Plant Mol. Biol. Rep. 25 98–114
Zhang CL, Shi H, Chen L, Wang X, Lü B, Zhang S, Liang Y, Liu R, Qian J, Sun W, You Z and Dong HS 2011 Harpin-induced expression and transgenic overexpression of the phloem protein gene AtPP2-A1 in Arabidopsis repress phloem feeding of the green peach aphid Myzus persicae. BMC Plant Biol. 11 doi:10.1186/1471-2229-11-11
Zhang SJ, Yang X, Sun MW, Sun F, Deng S and Dong HS 2009 Riboflavin-induced priming for pathogen defense in Arabidopsis thaliana. J. Integr. Plant Biol. 51 167–174
Zheng H, Rowland O and Kunst L 2005 Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell 17 1467–1481
Zitter TA and Beer SV 1998 Harpin for insect control. Phytopathology 88 S104–S105
Acknowledgements
We thank the graduate students studying in our lab for their assistance in aphid investigations. This study was supported by grants (2009ZX08002-004B and 2008ZX08002-001) from the National Novel Transgenic Organisms Breeding Project in China.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Lü B Sun W, Zhang S, Zhang C, Qian J, Wang X, Gao R and Dong H 2010 HrpNEa-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana. J. Biosci. 36 0000–0000] DOI
Beibei Lü Weiwei Sun and Shuping Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lü, B., Sun, W., Zhang, S. et al. HrpNEa-induced deterrent effect on phloem feeding of the green peach aphid Myzus persicae requires AtGSL5 and AtMYB44 genes in Arabidopsis thaliana . J Biosci 36, 123–137 (2011). https://doi.org/10.1007/s12038-011-9016-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-011-9016-2