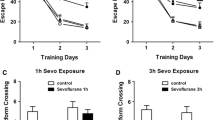
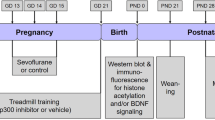
Abstract
Repeated neonatal exposures to sevoflurane induce long-term cognitive impairment that has been reported to have sex-dependent differences. Exercise promotes learning and memory by releasing lactate from the muscle. The study tested the hypothesis that lactate may improve long-term cognitive impairment induced by repeated neonatal exposures to sevoflurane through SIRT1-mediated regulation of adult hippocampal neurogenesis and synaptic plasticity. C57BL/6 mice of both genders were exposed to 3% sevoflurane for 2 h daily from postnatal day 6 (P6) to P8. In the intervention experiments, mice received lactate at 1 g/kg intraperitoneally once daily from P21 to P41. Behavioral tests including open field (OF), object location (OL), novel object recognition (NOR), and fear conditioning (FC) tests were performed to assess cognitive function. The number of 5-Bromo-2′- deoxyuridine positive (BrdU+) cells and BrdU+/DCX+ (doublecortin) co-labeled cells, expressions of brain-derived neurotrophic factor (BDNF), activity-regulated cytoskeletal-associated protein (Arc), early growth response 1 (Egr-1), SIRT1, PGC-1α and FNDC5, and long-term potentiation (LTP) were evaluated in the hippocampus. Repeated exposures to sevoflurane induced deficits in OL, NOR and contextual FC tests in male but not female mice. Similarly, adult hippocampal neurogenesis, synaptic plasticity-related proteins and hippocampal LTP were impaired after repeated exposures to sevoflurane in male but not female mice, which could rescue by lactate treatment. Our study suggests that repeated neonatal exposures to sevoflurane inhibit adult hippocampal neurogenesis and induce defects of synaptic plasticity in male but not female mice, which may contribute to long-term cognitive impairment. Lactate treatment rescues these abnormalities through activation of SIRT1.










Similar content being viewed by others
Data Availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882. https://doi.org/10.1523/JNEUROSCI.23-03-00876.2003
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118(3):502–515. https://doi.org/10.1097/ALN.0b013e3182834d77
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Wang C (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230. https://doi.org/10.1016/j.ntt.2011.01.001
Alvarado MC, Murphy KL, Baxter MG (2017) Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Brit J Anaesth 119(3):517–523. https://doi.org/10.1093/bja/aew473
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Hu D, Voigt RG, Paule MG, Chelonis JJ, Flick RP (2018) Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 129(1):89–105. https://doi.org/10.1097/ALN.0000000000002232
Schaefer ML, Perez PJ, Wang M, Gray C, Krall C, Sun X, Hunter E, Skinner J, Johns RA (2020) Neonatal Isoflurane Anesthesia or Disruption of Postsynaptic Density-95 Protein Interactions Change Dendritic Spine Densities and Cognitive Function in Juvenile Mice. Anesthesiology 133(4):812–823. https://doi.org/10.1097/ALN.0000000000003482
Fan XY, Shi G, Zhao P (2021) Neonatal Sevoflurane Exposure Impairs Learning and Memory by the Hypermethylation of Hippocampal Synaptic Genes. Mol Neurobiol 58(3):895–904. https://doi.org/10.1007/s12035-020-02161-4
Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L, Li M, Yang G, Dong Y, Zhang Y, Haas W, Xie Z (2020) Tau Contributes to Sevoflurane-induced Neurocognitive Impairment in Neonatal Mice. Anesthesiology 133(3):595–610. https://doi.org/10.1097/ALN.0000000000003452
Ji MH, Wang ZY, Sun XR, Tang H, Zhang H, Jia M, Qiu LL, Zhang GF, Peng YG, Yang JJ (2017) Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Mol Neurobiol 54(5):3759–3770. https://doi.org/10.1007/s12035-016-9943-x
Shu LJ, Du CF (2022) PHLDA1 promotes sevoflurane-induced pyroptosis of neuronal cells in developing rats through TRAF6-mediated activation of Rac1. Neurotoxicology 93:140–151. https://doi.org/10.1016/j.neuro.2022.09.007
Dai J, Li X, Wang C, Gu SX, Dai L, Zhang JY, Fan YX, Wu J (2021) Repeated neonatal sevoflurane induced neurocognitive impairment through NF-kappa B-mediated pyroptosis. J Neuroinflammation 18(1):180. https://doi.org/10.1186/s12974-021-02233-9
Sun Z, Satomoto M, Adachi YU, Kinoshita H, Makita K (2016) Inhibiting NADPH oxidase protects against long-term memory impairment induced by neonatal sevoflurane exposure in mice. Brit J Anaesth 117(1):80–86. https://doi.org/10.1093/bja/aew064
Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ (2016) Role of histone acetylation in long-term neurobehavioral effects of neonatal Exposure to sevoflurane in rats. Neurobiol Dis 91:209–220. https://doi.org/10.1016/j.nbd.2016.03.017
Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J (1995) Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A 92(25):11879–11883. https://doi.org/10.1073/pnas.92.25.11879
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. https://doi.org/10.1016/j.neuron.2011.05.001
Gage FH (2019) Adult neurogenesis in mammals. Science 364(6443):827–828. https://doi.org/10.1126/science.aav6885
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 22(4):589-599.e5. https://doi.org/10.1016/j.stem.2018.03.015
Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ (2009) A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325(5937):210–213. https://doi.org/10.1126/science.1173215
Danielson NB, Kaifosh P, Zaremba JD, Lovett-Barron M, Tsai J, Denny CA, Balough EM, Goldberg AR, Drew LJ, Hen R, Losonczy A, Kheirbek MA (2016) Distinct Contribution of Adult-Born Hippocampal Granule Cells to Context Encoding. Neuron 90(1):101–112. https://doi.org/10.1016/j.neuron.2016.02.019
Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S (2012) Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149(1):188–201. https://doi.org/10.1016/j.cell.2012.01.046
Miller SM, Sahay A (2019) Functions of adult-born neurons in hippocampal memory interference and indexing. Nat Neurosci 22(10):1565–1575. https://doi.org/10.1038/s41593-019-0484-2
Chi SP, Cui YX, Wang HP, Jiang JH, Zhang TX, Sun SH, Zhou Z, Zhong Y, Xiao BL (2022) Astrocytic Piezo1-mediated mechanotransduction determines adult neurogenesis and cognitive functions. Neuron 110(18):2984-2999.e8. https://doi.org/10.1016/j.neuron.2022.07.010
Berchtold NC, Castello N, Cotman CW (2010) Exercise and Time-Dependent Benefits To Learning and Memory. Neuroscience 167(3):588–597. https://doi.org/10.1016/j.neuroscience.2010.02.050
van Praag H, Christie BR, Sejnowski TJ, Gage FH (1999) Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 96(23):13427–13431. https://doi.org/10.1073/pnas.96.23.13427
Lz E, Lu JH, Selfridge JE, Burns JM, Swerdlow RH (2013) Lactate administration reproduces specific brain and liver exercise-related changes. J Neurochem 127(1):91–100. https://doi.org/10.1111/jnc.12394
Quistorff B, Secher NH, Van Lieshout JJ (2008) Lactate fuels the human brain during exercise. Faseb J 22(10):3443–3449. https://doi.org/10.1096/fj.08-106104
Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-lactate a novel signaling molecule in the brain? J Cerebr Blood F Met 35(7):1069–1075. https://doi.org/10.1038/jcbfm.2015.77
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 144(5):810–823. https://doi.org/10.1016/j.cell.2011.02.018
Yang JY, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. P Natl Acad Sci USA 111(33):12228–12233. https://doi.org/10.1073/pnas.1322912111
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D (2004) Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell 16(1):93–105. https://doi.org/10.1016/j.molcel.2004.08.031
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305(5682):390–392. https://doi.org/10.1126/science.1099196
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14(5):661–673. https://doi.org/10.1016/j.devcel.2008.02.004
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107(2):137–148. https://doi.org/10.1016/s0092-8674(01)00524-4
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379. https://doi.org/10.1073/pnas.0712145105
Iwahara N, Hisahara S, Hayashi T, Horio Y (2009) Transcriptional activation of NAD+-dependent protein deacetylase SIRT1 by nuclear receptor TLX. Biochem Biophys Res Commun 386(4):671–675. https://doi.org/10.1016/j.bbrc.2009.06.103
Fujita Y, Yamashita T (2018) Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front Neurosci 12:778. https://doi.org/10.3389/fnins.2018.00778
Williams EO, Taylor AK, Bell EL, Lim R, Kim DM, Guarente L (2016) Sirtuin 1 Promotes Deacetylation of Oct4 and Maintenance of Naive Pluripotency. Cell Rep 17(3):809–820. https://doi.org/10.1016/j.celrep.2016.09.046
Michan S, Li Y, Chou MMH, Parrella E, Ge HY, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LEH, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J Neurosci 30(29):9695–9707. https://doi.org/10.1523/Jneurosci.0027-10.2010
El Hayek L, Khalifeh M, Zibara V, Abi Assaad R, Emmanuel N, Karnib N, El-Ghandour R, Nasrallah P, Bilen M, Ibrahim P, Younes J, Abou Haidar E, Barmo N, Jabre V, Stephan JS, Sleiman SF (2019) Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci 39(13):2369–2382. https://doi.org/10.1523/JNEUROSCI.1661-18.2019
Carrard A, Casse F, Carron C, Burlet-Godinot S, Toni N, Magistretti PJ, Martin JL (2021) Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol Psychiatry 26(11):6723–6735. https://doi.org/10.1038/s41380-021-01122-0
Zhang JQ, Rong PJ, Zhang LJ, He H, Zhou T, Fan YH, Mo L, Zhao QY, Han Y, Li SY, Wang YF, Yan W, Chen HF, You ZL (2021) IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv 7(12):eabb9888. https://doi.org/10.1126/sciadv.abb9888
Carrard A, Elsayed M, Margineanu M, Boury-Jamot B, Fragniere L, Meylan EM, Petit JM, Fiumelli H, Magistretti PJ, Martin JL (2018) Peripheral administration of lactate produces antidepressant-like effects. Mol Psychiatry 23(2):392–399. https://doi.org/10.1038/mp.2016.179
Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX, Yang JJ (2016) NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51:109–118. https://doi.org/10.1016/j.bbi.2015.08.002
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise Induces Hippocampal BDNF through a PGC-1 alpha/FNDC5 Pathway. Cell Metab 18(5):649–659. https://doi.org/10.1016/j.cmet.2013.09.008
Wrann CD (2015) FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast 1(1):55–61. https://doi.org/10.3233/BPL-150019
Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJJ, Li GH, Sun LS (2012) Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics 130(3):E476–E485. https://doi.org/10.1542/peds.2011-3822
Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW (2014) Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology 83:9–17. https://doi.org/10.1016/j.neuropharm.2014.03.011
Boscolo A, Ori C, Bennett J, Wiltgen B, Jevtovic-Todorovic V (2013) Mitochondrial protectant pramipexole prevents sex-specific long-term cognitive impairment from early anaesthesia exposure in rats. Brit J Anaesth 110:47–52. https://doi.org/10.1093/bja/aet073
Wali B, Sayeed I, Stein DG, Raper J (2022) Prophylactic progesterone prevents adverse behavioural and neurocognitive effects of neonatal anaesthesia exposure in rat. Brit J Anaesth 128(2):301–310. https://doi.org/10.1016/j.bja.2021.10.030
Russell JMS, Chinn GA, Maharjan D, Eichbaum Y, Sall JW (2019) Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Brit J Anaesth 122(4):490–499. https://doi.org/10.1016/j.bja.2018.12.008
Tsai HW, Grant PA, Rissman EF (2009) Sex differences in histone modifications in the neonatal mouse brain. Epigenetics-Us 4(1):47–53. https://doi.org/10.4161/epi.4.1.7288
Karnib N, El-Ghandour R, El Hayek L, Nasrallah P, Khalifeh M, Barmo N, Jabre V, Ibrahim P, Bilen M, Stephan JS, Holson EB, Ratan RR, Sleiman SF (2019) Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacol 44(6):1152–1162. https://doi.org/10.1038/s41386-019-0313-z
Roumes H, Dumont U, Sanchez S, Mazuel L, Blanc J, Raffard G, Chateil JF, Pellerin L, Bouzier-Sore AK (2021) Neuroprotective role of lactate in rat neonatal hypoxia-ischemia. J Cerebr Blood F Met 41(2):342–358. https://doi.org/10.1177/0271678x20908355
Zhai XL, Li JY, Li LY, Sun Y, Zhang XN, Xue Y, Lv JX, Gao Y, Li SX, Yan W, Yin SM, Xiao ZY (2020) L-lactate preconditioning promotes plasticity-related proteins expression and reduces neurological deficits by potentiating GPR81 signaling in rat traumatic brain injury model. Brain Res 1746:146945. https://doi.org/10.1016/j.brainres.2020.146945
Berdugo-Vega G, Arias-Gil G, Lopez-Fernandez A, Artegiani B, Wasielewska JM, Lee CC, Lippert MT, Kempermann G, Takagaki K, Calegari F (2020) Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat Commun 11(1):135. https://doi.org/10.1038/s41467-019-14026-z
Stone SSD, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW (2011) Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 21(12):1348–1362. https://doi.org/10.1002/hipo.20845
Aimone JB, Deng W, Gage FH (2010) Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci 14(7):325–337. https://doi.org/10.1016/j.tics.2010.04.003
Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE (2018) Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science 361(6406):eaan8821. https://doi.org/10.1126/science.aan8821
Xia SH, Hu SW, Ge DG, Liu D, Wang D, Zhang S, Zhang Q, Yuan L, Li YQ, Yang JX, Wu P, Zhang HX, Han MH, Ding HL, Cao JL (2020) Chronic Pain Impairs Memory Formation via Disruption of Neurogenesis Mediated by Mesohippocampal Brain-Derived Neurotrophic Factor Signaling. Biol Psychiat 88(8):597–610. https://doi.org/10.1016/j.biopsych.2020.02.013
Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R (2009) Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 110(4):834–848. https://doi.org/10.1097/ALN.0b013e31819c463d
Huang J, Jing S, Chen X, Bao XH, Du ZY, Li H, Yang TD, Fan XT (2016) Propofol Administration During Early Postnatal Life Suppresses Hippocampal Neurogenesis. Mol Neurobiol 53(2):1031–1044. https://doi.org/10.1007/s12035-014-9052-7
Lev-Vachnish Y, Cadury S, Rotter-Maskowitz A, Feldman N, Roichman A, Illouz T, Varvak A, Nicola R, Madar R, Okun E (2019) L-Lactate Promotes Adult Hippocampal Neurogenesis. Front Neurosci 13:403. https://doi.org/10.3389/fnins.2019.00403
Liang XL, Zhang Y, Zhang C, Tang CC, Wang Y, Ren JJ, Chen X, Zhang Y, Zhu ZQ (2017) Effect of repeated neonatal sevoflurane exposure on the learning, memory and synaptic plasticity at juvenile and adult age. Am J Transl Res 9(11):4974–4983
Wan J, Shen CM, Wang Y, Wu QZ, Wang YL, Liu Q, Sun YM, Cao JP, Wu YQ (2021) Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood. Brain Res Bull 169:63–72. https://doi.org/10.1016/j.brainresbull.2021.01.007
Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB (2014) Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem 116:46–58. https://doi.org/10.1016/j.nlm.2014.08.004
Hsieh J, Gage FH (2005) Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol 17(6):664–671. https://doi.org/10.1016/j.ceb.2005.09.002
Kang SK, Cha SH, Jeon HG (2006) Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 15(2):165–174. https://doi.org/10.1089/scd.2006.15.165
Zhang HL, Zhao B, Han W, Sun YB, Yang P, Chen YJ, Ni D, Zhang J, Yin DM (2021) Acetylation of calmodulin regulates synaptic plasticity and fear learning. J Biol Chem 297(3):101034. https://doi.org/10.1016/j.jbc.2021.101034
Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou ZQ, Sun R, Luo AL, Li SY (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-kappa B pathway in neonatal mice. J Nutr Biochem 90:108579. https://doi.org/10.1016/j.jnutbio.2020.108579
Ma LH, Wan J, Yan J, Wang N, Liu YP, Wang HB, Zhou CH, Wu YQ (2022) Hippocampal SIRT1-Mediated Synaptic Plasticity and Glutamatergic Neuronal Excitability Are Involved in Prolonged Cognitive Dysfunction of Neonatal Rats Exposed to Propofol. Mol Neurobiol 59(3):1938–1953. https://doi.org/10.1007/s12035-021-02684-4
Taliaz D, Stall N, Dar DE, Zangen A (2010) Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatr 15(1):80–92. https://doi.org/10.1038/mp.2009.67
Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356. https://doi.org/10.1016/j.expneurol.2004.11.016
Wang CS, Kavalali ET, Monteggia LM (2022) BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell 185(1):62–76. https://doi.org/10.1016/j.cell.2021.12.003
Acknowledgements
The authors thank Yiquan Wei and Li Liu for their laboratory assistance and other laboratory members
for the study-related discussion.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82071196, 82271216, and 82001153), and by the Natural Science Foundation of Jiangsu Province (BK20221463).
Author information
Authors and Affiliations
Contributions
Li–Li Qiu performed neonatal anesthesia, drug treatment, Western blot experiment, long-term potentiation recordings and drafted the manuscript. Xiao-Xiang Tan, Jiao-Jiao Yang, Mu-Huo Ji and Hui Zhang performed behavioral tests and Immunofluorescence. Chunjie Zhao performed the statistical analysis. Jiang-Yan Xia and Jie Sun designed the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental procedures were approved by the Laboratory Animal Care and Use Committee of Southeast University (Ethical permission code: 20210301071).
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to the publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, LL., Tan, XX., Yang, JJ. et al. Lactate Improves Long-term Cognitive Impairment Induced By Repeated Neonatal Sevoflurane Exposures Through SIRT1-mediated Regulation of Adult Hippocampal Neurogenesis and Synaptic Plasticity in Male Mice. Mol Neurobiol 60, 5273–5291 (2023). https://doi.org/10.1007/s12035-023-03413-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03413-9