Abstract
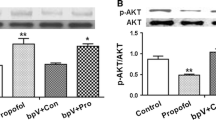
Neonates who receive repeated or prolonged general anesthesia before the age of 4 are at a significantly higher risk of developing cognitive dysfunction later in life. In this study, we investigated the effects of repeated neonatal propofol exposure on hippocampal synaptic plasticity, neuronal excitability, and cognitive function. Adeno-associated SIRT1 virus with CaMKIIɑ promotor and a viral vector carrying the photosensitive gene ChR2 with the CaMKIIɑ promotor, as well as their control vectors, were stereotaxically injected into the hippocampal CA1 region of postnatal day 5 (PND-5) rats. PND-7 rats were given intraperitoneal injection of 60 mg/kg propofol or fat emulsion for three consecutive days. Western blotting, Golgi staining, and double immunofluorescence staining were used to evaluate the SIRT1 expression, synaptic plasticity, and the excitability of neurons in the hippocampal CA1 region. The Morris water maze (MWM) test was conducted on PND-30 to assess the learning and memory abilities of rats. Repeated neonatal propofol exposure reduced SIRT1 expression, suppressed synaptic plasticity, decreased glutamatergic neuron excitability in the hippocampus, and damaged learning and memory abilities. Overexpression of SIRT1 attenuated propofol-induced cognitive dysfunction, excitation-inhibition imbalance, and synaptic plasticity damage. After optogenetic stimulation of glutamatergic neurons in the hippocampal CA1 region, the learning and memory abilities of rats exposed to propofol were improved on PND-30. Our findings demonstrate that SIRT1 plays an important role in cognitive dysfunction induced by repeated neonatal propofol exposure by suppressing synaptic plasticity and neuronal excitability.









Similar content being viewed by others
Availability of Data and Materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Code Availability
Not applicable.
References
Kuratani N (2015) The cutting edge of neonatal anesthesia: the tide of history is changing. J Anesth 29(1):1–3. https://doi.org/10.1007/s00540-014-1817-7
Franks NP, Lieb WR (1994) Molecular and cellular mechanisms of general anaesthesia. Nature 367(6464):607–614. https://doi.org/10.1038/367607a0
DeFrances CJ, Cullen KA, Kozak LJ (2007) National hospital discharge survey: 2005 annual summary with detailed diagnosis and procedure data. Vital and health statistics Series 13, Data from the National Health Survey (165):1-209
McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, Grobler A, Stargatt R et al (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393(10172):664–677. https://doi.org/10.1016/s0140-6736(18)32485-1
Sun LS, Li G, DiMaggio CJ, Byrne MW, Ing C, Miller TL, Bellinger DC, Han S et al (2012) Feasibility and pilot study of the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) project. J Neurosurg Anesthesiol 24(4):382–388. https://doi.org/10.1097/ANA.0b013e31826a0371
Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, Buenvenida SL, Gleich SJ et al (2018) Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 129(1):89–105. https://doi.org/10.1097/aln.0000000000002232
Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110(4):796–804. https://doi.org/10.1097/01.anes.0000344728.34332.5d
Bosnjak ZJ, Logan S, Liu Y, Bai X (2016) Recent insights into molecular mechanisms of propofol-induced developmental neurotoxicity: implications for the protective strategies. Anesth Analg 123(5):1286–1296. https://doi.org/10.1213/ane.0000000000001544
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882. https://doi.org/10.1523/jneurosci.23-03-00876.2003
Kletecka J, Holeckova I, Brenkus P, Pouska J, Benes J, Chytra I (2019) Propofol versus sevoflurane anaesthesia: effect on cognitive decline and event-related potentials. J Clin Monit Comput 33(4):665–673. https://doi.org/10.1007/s10877-018-0213-5
Nishiyama J (2019) Plasticity of dendritic spines: molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin Neurosci 73(9):541–550. https://doi.org/10.1111/pcn.12899
Tachibana K, Hashimoto T, Kato R, Tsuruga K, Ito R, Morimoto Y (2011) Long-lasting effects of neonatal pentobarbital administration on spatial learning and hippocampal synaptic plasticity. Brain Res 1388:69–76. https://doi.org/10.1016/j.brainres.2011.02.086
Cheyne JE, Montgomery JM (2020) The cellular and molecular basis of in vivo synaptic plasticity in rodents. Am J Physiol Cell Physiol 318(6):C1264-c1283. https://doi.org/10.1152/ajpcell.00416.2019
Iascone DM, Li Y, Sümbül U, Doron M, Chen H, Andreu V, Goudy F, Segev I et al (2018) Whole-neuron synaptic mapping reveals local balance between excitatory and inhibitory synapse organization. J bioRxiv:395384. https://doi.org/10.1101/395384
Liu G (2004) Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 7(4):373–379. https://doi.org/10.1038/nn1206
Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81(3):471–483. https://doi.org/10.1016/j.neuron.2014.01.028
Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K et al (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30(29):9695–9707. https://doi.org/10.1523/jneurosci.0027-10.2010
Li XH, Chen C, Tu Y, Sun HT, Zhao ML, Cheng SX, Qu Y, Zhang S (2013) Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol 48(3):490–499. https://doi.org/10.1007/s12035-013-8437-3
Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D et al (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466(7310):1105–1109. https://doi.org/10.1038/nature09271
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711. https://doi.org/10.1146/annurev.neuro.23.1.649
Sugino T, Maruyama M, Tanno M, Kuno A, Houkin K, Horio Y (2010) Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett 584(13):2821–2826. https://doi.org/10.1016/j.febslet.2010.04.063
Yang P, Wang Z, Zhang Z, Liu D, Manolios EN, Chen C, Yan X, Zuo W et al (2018) The extended application of The Rat Brain in Stereotaxic Coordinates in rats of various body weight. J Neurosci Methods 307:60–69. https://doi.org/10.1016/j.jneumeth.2018.06.026
Sheng M, Greenberg ME (1990) The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4(4):477–485. https://doi.org/10.1016/0896-6273(90)90106-p
Shi Y, Hu D, Rodgers EL, Katusic SK, Gleich SJ, Hanson AC, Schroeder DR, Flick RP et al (2018) Epidemiology of general anesthesia prior to age 3 in a population-based birth cohort. Paediatr Anaesth 28(6):513–519. https://doi.org/10.1111/pan.13359
Langley MS, Heel RC (1988) Propofol. A review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anaesthetic. Drugs 35(4):334–372. https://doi.org/10.2165/00003495-198835040-00002
Picard P, Tramèr MR (2000) Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg 90(4):963–969. https://doi.org/10.1097/00000539-200004000-00035
Chidambaran V, Costandi A, D’Mello A (2015) Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs 29(7):543–563. https://doi.org/10.1007/s40263-015-0259-6
Smith MC, Williamson J, Yaster M, Boyd GJ, Heitmiller ES (2012) Off-label use of medications in children undergoing sedation and anesthesia. Anesth Analg 115(5):1148–1154. https://doi.org/10.1213/ANE.0b013e3182501b04
U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm. Accessed 27 April 2017
Olutoye OA, Baker BW, Belfort MA, Olutoye OO (2018) Food and Drug Administration warning on anesthesia and brain development: implications for obstetric and fetal surgery. Am J Obstet Gynecol 218(1):98–102. https://doi.org/10.1016/j.ajog.2017.08.107
Qiao H, Li Y, Xu Z, Li W, Fu Z, Wang Y, King A, Wei H (2017) Propofol affects neurodegeneration and neurogenesis by regulation of autophagy via effects on intracellular calcium homeostasis. Anesthesiology 127(3):490–501. https://doi.org/10.1097/aln.0000000000001730
Yu D, Jiang Y, Gao J, Liu B, Chen P (2013) Repeated exposure to propofol potentiates neuroapoptosis and long-term behavioral deficits in neonatal rats. Neurosci Lett 534:41–46. https://doi.org/10.1016/j.neulet.2012.12.033
Driessen J, Willems S, Dercksen S, Giele J, van der Staak F, Smeitink J (2007) Anesthesia-related morbidity and mortality after surgery for muscle biopsy in children with mitochondrial defects. Paediatr Anaesth 17(1):16–21. https://doi.org/10.1111/j.1460-9592.2006.02043.x
Wang YL, Chen X, Wang ZP (2015) Detrimental effects of postnatal exposure to propofol on memory and hippocampal LTP in mice. Brain Res 1622:321–327. https://doi.org/10.1016/j.brainres.2015.06.044
Nagashima K, Zorumski CF, Izumi Y (2005) Propofol inhibits long-term potentiation but not long-term depression in rat hippocampal slices. Anesthesiology 103(2):318–326. https://doi.org/10.1097/00000542-200508000-00015
Schaefer ML, Perez PJ, Wang M, Gray C, Krall C, Sun X, Hunter E, Skinner J et al (2020) Neonatal isoflurane anesthesia or disruption of postsynaptic density-95 protein interactions change dendritic spine densities and cognitive function in juvenile mice. Anesthesiology 133(4):812–823. https://doi.org/10.1097/aln.0000000000003482
Yu Y, Yang Y, Tan H, Boukhali M, Khatri A, Yu Y, Hua F, Liu L et al (2020) Tau contributes to sevoflurane-induced neurocognitive impairment in neonatal mice. Anesthesiology 133(3):595–610. https://doi.org/10.1097/aln.0000000000003452
Dai CL, Li H, Hu X, Zhang J, Liu F, Iqbal K, Gong CX (2020) Neonatal exposure to anesthesia leads to cognitive deficits in old age: prevention with intranasal administration of insulin in mice. Neurotox Res 38(2):299–311. https://doi.org/10.1007/s12640-020-00223-y
Perna RB, Loughan AR, Le JA, Hertza J (2015) Prenatal and perinatal anesthesia and the long-term cognitive sequelae: a review. Appl Neuropsychol Child 4(1):65–71. https://doi.org/10.1080/21622965.2013.779275
Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25(3):138–145. https://doi.org/10.1016/j.tem.2013.12.001
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5(3):344–352. https://doi.org/10.1002/emmm.201302451
Alves-Fernandes DK, Jasiulionis MG (2019) The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci 20(13). https://doi.org/10.3390/ijms20133153
Chen C, Zhou M, Ge Y, Wang X (2020) SIRT1 and aging related signaling pathways. Mech Ageing Dev 187:111215. https://doi.org/10.1016/j.mad.2020.111215
Tang X, Zhao Y, Zhou Z, Yan J, Zhou B, Chi X, Luo A, Li S (2020) Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in developing mice. Oxid Med Cell Longev 2020:9018624. https://doi.org/10.1155/2020/9018624
Skaper SD, Facci L, Zusso M, Giusti P (2017) Synaptic plasticity, dementia and Alzheimer disease. CNS Neurol Disord Drug Targets 16(3):220–233. https://doi.org/10.2174/1871527316666170113120853
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33(3):121–129. https://doi.org/10.1016/j.tins.2010.01.001
Bannon NM, Chistiakova M, Volgushev M (2020) Synaptic plasticity in cortical inhibitory neurons: what mechanisms may help to balance synaptic weight changes? Front Cell Neurosci 14:204. https://doi.org/10.3389/fncel.2020.00204
van Strien NM, Cappaert NL, Witter MP (2009) The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci 10(4):272–282. https://doi.org/10.1038/nrn2614
Funding
This work is supported by the National Natural Science Foundation of China (81971051, 82171191) and the Natural Science Foundation of Jiangsu Province (BK20191464).
Author information
Authors and Affiliations
Contributions
As the corresponding author, Yu-Qing Wu conceived the study, completed its design and coordination, and secured funding for the project. Under the guidance and supervision of Yu-Qing Wu and Cheng-Hua Zhou, Lin-Hui Ma performed the neonatal propofol anesthesia, sample preparation, Golgi-Cox staining, double immunofluorescence staining, and the optogenetic stimulation. Jie Wan completed the data collection and statistical analysis. Jing Yan was responsible for the reproduction and feeding of rats. Ning Wang performed the Western blot experiment. Yan-Ping Liu and Hai-Bi Wang completed the Morris water maze test. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the Ethics Committee of XuZhou Medical University.
Consent to Participate
Not applicable.
Consent for Publication
Yes.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, LH., Wan, J., Yan, J. et al. Hippocampal SIRT1-Mediated Synaptic Plasticity and Glutamatergic Neuronal Excitability Are Involved in Prolonged Cognitive Dysfunction of Neonatal Rats Exposed to Propofol. Mol Neurobiol 59, 1938–1953 (2022). https://doi.org/10.1007/s12035-021-02684-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02684-4