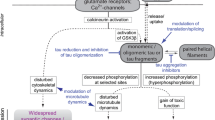
Abstract
A few protein kinases and phosphatases regulate tau protein phosphorylation and an imbalance in their enzyme activity results in tau hyper-phosphorylation. Aberrant tau phosphorylation causes tau to dissociate from the microtubules and clump together in the cytosol to form neurofibrillary tangles (NFTs), which lead to the progression of neurodegenerative disorders including Alzheimer’s disease (AD) and other tauopathies. Hence, targeting hyperphosphorylated tau protein is a restorative approach for treating neurodegenerative tauopathies. The cyclin-dependent kinase (Cdk5) and the glycogen synthase kinase (GSK3β) have both been implicated in aberrant tau hyperphosphorylation. The limited transport of drugs through the blood–brain barrier (BBB) for reaching the central nervous system (CNS) thus represents a significant problem in the development of drugs. Drug delivery systems based on nanocarriers help solve this problem. In this review, we discuss the tau protein, regulation of tau phosphorylation and abnormal hyperphosphorylation, drugs in use or under clinical trials, and treatment strategies for tauopathies based on the critical role of tau hyperphosphorylation in the pathogenesis of the disease.
Graphical Abstract
Pathology of neurodegenerative disease due to hyperphosphorylation and various therapeutic approaches including nanotechnology for its treatment.





Similar content being viewed by others
Data Availability
Not applicable as no datasets were generated or analysed during the current study.
Abbreviations
- NFTs:
-
Neurofibrillary tangles
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- Cdk5:
-
Cyclin-dependent kinase
- GSK3β:
-
Glycogen synthase kinase
- BBB:
-
Blood-brain barrier
- DALYs:
-
Disability-adjusted life-years
- MAPT:
-
Microtubule-associated protein tau FTDP-17, frontotemporal dementia associated with Parkinsonism having relation with chromosome 17
- PSP:
-
Progressive supranuclear palsy
- CBD:
-
Corticobasal degeneration
- PiD:
-
Pick’s disease
- AGD:
-
Argyrophilic grain disease
- GGT:
-
Globular glial tauopathy
- CNS:
-
Central nervous system
- pI:
-
Isoelectric point
- N:
-
Terminal, amino-terminal
- C:
-
Terminal, carboxy-terminal
- 3R:
-
C-terminal region with three microtubule-binding repeats of 31 or 32 amino acids
- 4R:
-
C-terminal region with four microtubule-binding repeats of 31 or 32 amino acids
- NMR:
-
Nuclear magnetic resonance
- PHFs:
-
Paired helical filaments
- Ser:
-
Serine
- Thr:
-
Threonine
- Tyr:
-
Tyrosine
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- MARK:
-
Microtubule affinity-regulating kinase
- PP1:
-
Protein phosphatase-1
- SFs:
-
Straight filaments
- Aβ:
-
β-Amyloid
- APP:
-
β-Amyloid precursor protein
- PS1:
-
Presenilin 1 gene
- PS2:
-
Presenilin 2 gene
- APOE:
-
Apolipoprotein E
- mTOR:
-
Mammalian target of rapamycin
- SNpc:
-
Substantia nigra pars compacta
- FTLD:
-
Frontotemporal lobar degeneration
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- Raptor:
-
Regulatory associated protein of mTOR
- mLST8:
-
Mammalian lethal with Sec13 protein 8
- PRAS40:
-
40 KDa proline-rich AKT substrate
- Deptor:
-
DEP-domain-containing mTOR-interacting protein
- Rictor:
-
Rapamycin-insensitive companion of mTOR
- mSIN1:
-
Mammalian stress-activated protein kinase interacting protein
- Protor-1:
-
Protein observed with Rictor-1
- HEAT:
-
Huntingtin, EF3, the A subunit of PP2A, TOR1
- 4EBPs:
-
EIF4E-binding proteins
- IL-2:
-
Interleukin-2
- TKIs:
-
Tyrosine kinase inhibitors
- EMA:
-
European Medicines Agency
- ADNP:
-
Activity-dependent neuroprotective protein
- TAI:
-
Tau aggregation inhibitor
- MTC:
-
Methylthioninium chloride
- LMT:
-
Leuco-methylthioninum
- LMTM:
-
Leucomethylthioninium bis-hydromethanesulfonate
- PPMT:
-
PP2A methyltransferase
- LCMT:
-
Leucine carboxyl methyl transferase
- PME:
-
PP2A methyl esterase
- SAD:
-
Single ascending dose
- CADRO:
-
Common Alzheimer’s and Related Dementias Research Ontology
- MOA:
-
Mechanisms of action
- DMTs:
-
Disease-modifying therapies
- CDR-SB:
-
Clinical Dementia Rating scale Sum of Boxes
References
Feigin VL, Nichols E, Alam T et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease study 2016. Lancet Neurol 18:459–480. https://doi.org/10.1016/S1474-4422(18)30499-X
Yiannopoulou KG, Papageorgiou SG (2020) Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis 12:117957352090739. https://doi.org/10.1177/1179573520907397
Kovacs GG (2018) Tauopathies. Handb Clin Neurol 145:355–368. https://doi.org/10.1016/B978-0-12-802395-2.00025-0
Saha P, Sen N (2019) Tauopathy: a common mechanism for neurodegeneration and brain aging. Mech Ageing Dev 178:72–79. https://doi.org/10.1016/j.mad.2019.01.007
Stepanov A, Karelina T, Markevich N et al (2018) A mathematical model of multisite phosphorylation of tau protein. PLoS ONE 13:1–17. https://doi.org/10.1371/journal.pone.0192519
Kumar A, Tan A, Wong J et al (2017) Nanotechnology for neuroscience: promising approaches for diagnostics, therapeutics and brain activity mapping. Adv Funct Mater 27:1–30. https://doi.org/10.1002/adfm.201700489
Kang YJ, Cutler EG, Cho H (2018) Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg 5. https://doi.org/10.1186/s40580-018-0168-8
Iqbal K, Liu F, Gong CX (2016) Tau and neurodegenerative disease: the story so far. Nat Rev Neurol 12:15–27. https://doi.org/10.1038/nrneurol.2015.225
Islam Khan R, Nirzhor SSR, Rashid B (2018) A closer look into the role of protein tau in the identification of promising therapeutic targets for Alzheimer’s disease. Brain Sci 8. https://doi.org/10.3390/brainsci8090162
Hernµndez F, Avila J (2007) Cell Mol Life Sci 64:2219–2233. https://doi.org/10.1007/s00018-007-7220-x
Chen K (2020) “Phosphorylation of the tau protein in neurodegenerative disease.” Biomed J Sci Tech Res 26. https://doi.org/10.26717/bjstr.2020.26.004393
Iqbal K, Liu F, Gong C-X, Grundke-Iqbal I (2010) Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7:656–664. https://doi.org/10.2174/156720510793611592
Cherry JD, Esnault CD, Baucom ZH et al (2021) Tau isoforms are differentially expressed across the hippocampus in chronic traumatic encephalopathy and Alzheimer’s disease. Acta Neuropathol Commun 9:1–17. https://doi.org/10.1186/s40478-021-01189-4
Gao Y-L, Wang N, Sun F-R, et al (2018) Tau in neurodegenerative disease. Ann Transl Med Vol 6, No 10 (May 2018) Ann Transl Med (Focus “Translational Neurodegener
Zhang W, Tarutani A, Newell KL et al (2020) Novel tau filament fold in corticobasal degeneration. Nature 580:283–287. https://doi.org/10.1038/s41586-020-2043-0
Kouri N, Whitwell JL, Josephs KA et al (2011) Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol 7:263–272. https://doi.org/10.1038/nrneurol.2011.43
Correia SC, Perry G, Moreira PI (2016) Mitochondrial traffic jams in Alzheimer’s disease - pinpointing the roadblocks. Biochim Biophys Acta - Mol Basis Dis 1862:1909–1917. https://doi.org/10.1016/j.bbadis.2016.07.010
Lim S, Haque MM, Kim D et al (2014) Cell-based models to investigate Tau aggregation. Comput Struct Biotechnol J 12:7–13. https://doi.org/10.1016/j.csbj.2014.09.011
Chun W, Johnson GVW (2007) Activation of Glycogen Synthase Kinase 3β Promotes the Intermolecular Association of Tau: the use of fluorescence resonance energy transfer microscopy *. 282:23410–23417. https://doi.org/10.1074/jbc.M703706200
Chun W, Waldo GS, Johnson GVW (2011) Chapter 9 split GFP complementation assay for quantitative. 670:109–123. https://doi.org/10.1007/978-1-60761-744-0
Oakley SS, Maina MB, Marshall KE, Al-hilaly YK (2020). Tau filament self-assembly and structure : tau as a therapeutic target tau filament self-assembly and structure : tau as a therapeutic target. https://doi.org/10.3389/fneur.2020.590754
Hanger DP, Anderton BH, Noble W (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15:112–119. https://doi.org/10.1016/j.molmed.2009.01.003
Dickey CA, Kamal A, Lundgren K et al (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117:648–658. https://doi.org/10.1172/JCI29715
Yan X, Uronen RL, Huttunen HJ (2020) The interaction of α-synuclein and tau: a molecular conspiracy in neurodegeneration? Semin Cell Dev Biol 99:55–64. https://doi.org/10.1016/j.semcdb.2018.05.005
Barbier P, Zejneli O, Martinho M et al (2019) Role of tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci 10:1–14. https://doi.org/10.3389/fnagi.2019.00204
Drummond E, Pires G, Macmurray C, et al (2020) Phosphorylated tau interactome in the human Alzheimer ’ s disease brain. 1–15. https://doi.org/10.1093/brain/awaa223
Sergeant N, Bretteville A, Hamdane M et al (2008) Biochemistry of tau in Alzheimer’s disease and related neurological disorders. Expert Rev Proteomics 5:207–224. https://doi.org/10.1586/14789450.5.2.207
Arendt T, Stieler JT, Holzer M (2016) Tau and tauopathies. Brain Res Bull 126:238–292. https://doi.org/10.1016/j.brainresbull.2016.08.018
Noble W, Hanger DP, Miller CCJ, Lovestone S (2013) The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol 4 JUL:1–11. https://doi.org/10.3389/fneur.2013.00083
Kaidanovich-Beilin O, Woodgett JR (2011) GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci 4:1–25. https://doi.org/10.3389/fnmol.2011.00040
Sutherland C (2011) What are the bona fide GSK3 substrates? Int J Alzheimers Dis 2011. https://doi.org/10.4061/2011/505607
Dolan PJ, Johnson GVW (2011) The role of tau kinases in Alzheimer’s disease 13:595–603
Virshup DM, Shenolikar S (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33:537–545. https://doi.org/10.1016/j.molcel.2009.02.015
Alquezar C, Arya S, Kao AW (2021) Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front Neurol 11:1–24. https://doi.org/10.3389/fneur.2020.595532
Lei P, Ayton S, Bush AI, Adlard PA (2011) GSK-3 in neurodegenerative diseases. Int J Alzheimers Dis 2011. https://doi.org/10.4061/2011/189246
Ao C, Li C, Chen J et al (2022) The role of Cdk5 in neurological disorders. Front Cell Neurosci 16:1–13. https://doi.org/10.3389/fncel.2022.951202
Jouanne M, Rault S, Voisin-Chiret AS (2017) Tau protein aggregation in Alzheimer’s disease: an attractive target for the development of novel therapeutic agents. Eur J Med Chem 139:153–167. https://doi.org/10.1016/j.ejmech.2017.07.070
Domise M, Didier S, Marinangeli C, et al (2016) AMP-activated protein kinase modulates tau phosphorylation and tau pathology in vivo. Nat Publ Gr 1–12. https://doi.org/10.1038/srep26758
Lund H, Gustafsson E, Svensson A, et al (2014) MARK4 and MARK3 associate with early tau phosphorylation in Alzheimer ’ s disease granulovacuolar degeneration bodies. 1–15
Wu JW, Hussaini SA, Bastille IM, et al (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 1–11. https://doi.org/10.1038/nn.4328
Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell *. 284:12845–12852. https://doi.org/10.1074/jbc.M808759200
Sonawane SK, Chinnathambi S (2018) Prion-like propagation of post-translationally modified tau in Alzheimer’s disease : a hypothesis
Fontaine SN, Sabbagh JJ, Baker J et al (2015) Cellular factors modulating the mechanism of tau protein aggregation. Cell Mol Life Sci 72:1863–1879. https://doi.org/10.1007/s00018-015-1839-9
Caillet-Boudin ML, Buée L, Sergeant N, Lefebvre B (2015) Regulation of human MAPT gene expression. Mol Neurodegener 10:1–14. https://doi.org/10.1186/s13024-015-0025-8
Souter S, Lee G (2009) Microtubule-associated protein tau in human prostate cancer cells: isoforms, phosphorylation, and interactions. J Cell Biochem 108:555–564. https://doi.org/10.1002/jcb.22287
Barron MR, Gartlon J, Dawson LA et al (2020) Increasing tau 4R tau levels exacerbates hippocampal tau hyperphosphorylation in the hTau model of tauopathy but also tau dephosphorylation following acute systemic inflammation. Front Immunol 11:293. https://doi.org/10.3389/fimmu.2020.00293
Pascual G, Wadia JS, Zhu X et al (2017) Immunological memory to hyperphosphorylated tau in asymptomatic individuals. Acta Neuropathol 133:767–783. https://doi.org/10.1007/s00401-017-1705-y
Zhang Y, Thompson R, Zhang H, Xu H (2011) APP processing in Alzheimer’s disease. 1–13
Karran E, Mercken M, De SB (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10:698–712. https://doi.org/10.1038/nrd3505
Myers A, McGonigle P (2019) Overview of transgenic mouse models for Alzheimer’s disease. Curr Protoc Neurosci 89:1–21. https://doi.org/10.1002/cpns.81
Bloom GS (2014) Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 71:505–508. https://doi.org/10.1001/jamaneurol.2013.5847
Deture MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:1–18. https://doi.org/10.1186/s13024-019-0333-5
Chen GF, Xu TH, Yan Y et al (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38:1205–1235. https://doi.org/10.1038/aps.2017.28
Kametani F, Hasegawa M (2018) Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front Neurosci 12. https://doi.org/10.3389/fnins.2018.00025
Morley JE, Farr SA, Nguyen AD, Xu F (2019) What is the physiological function of amyloid-beta protein? J Nutr Heal Aging 23:225–226. https://doi.org/10.1007/s12603-019-1162-5
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Blennow K, Zetterberg H (2018) Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med 284:643–663
Canosa A, Pagani M, Brunetti M et al (2019) Correlation between apolipoprotein E genotype and brain metabolism in amyotrophic lateral sclerosis. Eur J Neurol 26:306–312. https://doi.org/10.1111/ene.13812
Mucke L (2009) Neuroscience: Alzheimer’s disease. Nature 461:895–897. https://doi.org/10.1038/461895a
Hou Y, Dan X, Babbar M et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15:565–581. https://doi.org/10.1038/s41582-019-0244-7
Onyike CU, Diehl-schmid J (2013) The epidemiology of frontotemporal dementia 25:130–137. https://doi.org/10.3109/09540261.2013.776523
Nichols E, Steinmetz JD, Vollset SE et al (2022) Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease study 2019. Lancet Public Heal 7:e105–e125
Valdez C, Ysselstein D, Young TJ et al (2020) Progranulin mutations result in impaired processing of prosaposin and reduced glucocerebrosidase activity. Hum Mol Genet 29:716–726. https://doi.org/10.1093/hmg/ddz229
Gijselinck I, Van LT, Van Der ZJ et al (2012) A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum : a gene identifi cation study. Lancet Neurol 11:54–65. https://doi.org/10.1016/S1474-4422(11)70261-7
Weihl CC, Pestronk A, Kimonis VE (2009) Neuromuscular disorders valosin-containing protein disease : inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord 19:308–315. https://doi.org/10.1016/j.nmd.2009.01.009
Bigio EH Making the Diagnosis of Frontotemporal Lobar Degeneration. https://doi.org/10.5858/arpa.2012-0075-RA
Gasparini L, Terni B, Spillantini MG (2007) Frontotemporal dementia with tau pathology. Neurodegener Dis 4:236–253. https://doi.org/10.1159/000101848
Liu M-N, Lau C-I, Lin C-P (2019) Precision medicine for frontotemporal dementia. Front psychiatry 10:75. https://doi.org/10.3389/fpsyt.2019.00075
Wszolek ZK, Tsuboi Y, Ghetti B et al (2006) Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Orphanet J Rare Dis 1:1–9. https://doi.org/10.1186/1750-1172-1-30
Bang J, Spina S, Miller BL (2018) Non-Alzheimer’s dementia 1. 386:1672–1682. https://doi.org/10.1016/S0140-6736(15)00461-4.Non-Alzheimer
R. C, A. K, J.A. K, (2010) Frontotemporal dementia: a review for primary care physicians. Am Fam Physician 82:1372–1377
Pippin MM, Gupta V (2022) Pick disease. In StatPearls. StatPearls Publishing
Bang J, Spina S, Miller BL (2015) Frontotemporal dementia. Lancet 386:1672–1682. https://doi.org/10.1016/S0140-6736(15)00461-4
Viscidi E, Litvan I, Dam T et al (2021) Clinical features of patients with progressive supranuclear palsy in an US insurance claims database 12:1–10. https://doi.org/10.3389/fneur.2021.571800
Dickson DW, Rademakers R, Hutton ML (2007) Progressive supranuclear palsy: pathology and genetics. Brain Pathol 17:74–82. https://doi.org/10.1111/j.1750-3639.2007.00054.x
Kovacs GG, Lukic MJ, Irwin DJ et al (2020) Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol 140:99–119. https://doi.org/10.1007/s00401-020-02158-2
Boeve BF (2012) Progressive supranuclear palsy Park Relat Disord 18:S192–S194. https://doi.org/10.1016/s1353-8020(11)70060-8
Shoeibi A, Olfati N, Litvan I (2018) Preclinical, phase I, and phase II investigational clinical trials for treatment of progressive supranuclear palsy. Expert Opin Investig Drugs 27:349–361. https://doi.org/10.1080/13543784.2018.1460356
Day GS, Sung Lim T, Hassenstab J et al (2017) Differentiating cognitive impairment due to corticobasal degeneration and Alzheimer disease conclusions: CBD may mimic AD dementia early in its disease course. Interval screening for Neurology 88:1273–1281
Armstrong MJ (2014) Diagnosis and treatment of corticobasal degeneration topical collection on movement disorders. Curr Treat Options Neurol 16. https://doi.org/10.1007/s11940-013-0282-1
Ikeda C, Yokota O, Miki T, et al (2018) Astrocytic tau pathologies in argyrophilic grain disease and related four-repeat tauopathies. Acta Med Okayama 72:211–221. https://doi.org/10.18926/AMO/56066
Yokota O, Miki T, Ikeda C et al (2018) Neuropathological comorbidity associated with argyrophilic grain disease. Neuropathology 38:82–97. https://doi.org/10.1111/neup.12429
Sengoku R (2020) Aging and Alzheimer’s disease pathology. Neuropathology 40:22–29. https://doi.org/10.1111/neup.12626
Kabir MT, Uddin MS, Begum MM et al (2019) Cholinesterase inhibitors for Alzheimer’s disease: multitargeting strategy based on anti-Alzheimer’s drugs repositioning. Curr Pharm Des 25:3519–3535. https://doi.org/10.2174/1381612825666191008103141
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Bill F, Foundation MG (2016) Articles global, regional, and national burden of Parkinson’s disease, 1990 – 2016 : a systematic analysis for the Global Burden of Disease study 2016. 939–953. https://doi.org/10.1016/S1474-4422(18)30295-3
Wanneveich M, Elbaz A, Joly P, Society MD (2018) Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010–2030) in France. 00:1–7. https://doi.org/10.1002/mds.27447
Gao H, Hong J (2011) Progress in neurobiology gene – environment interactions : key to unraveling the mystery of Parkinson’s disease. Prog Neurobiol 94:1–19. https://doi.org/10.1016/j.pneurobio.2011.03.005
Verstraeten A, Theuns J, Van BC (2015) Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31:140–149. https://doi.org/10.1016/j.tig.2015.01.004
Cannon JR, Greenamyre JT (2013) Neurobiology of disease gene – environment interactions in Parkinson ’ s disease : Speci fi c evidence in humans and mammalian models. Neurobiol Dis 57:38–46. https://doi.org/10.1016/j.nbd.2012.06.025
Marras C, Canning CG, Goldman SM (2019) Environment, lifestyle, and Parkinson’s disease : implications for prevention in the next decade. 1–11. https://doi.org/10.1002/mds.27720
Ball N, Teo W, Chandra S, et al (2019) Parkinson’s disease and the Environment. 10. https://doi.org/10.3389/fneur.2019.00218
Galloway PG, Grundke-Iqbal I, Iqbal K, Perry G (1988) Lewy bodies contain epitopes both shared and distinct from Alzheimer neurofibrillary tangles. J Neuropathol Exp Neurol 47:654–663. https://doi.org/10.1097/00005072-198811000-00008
Zhang X, Gao F, Wang D et al (2018) Tau pathology in Parkinson’s disease. Front Neurol 9:1–7. https://doi.org/10.3389/fneur.2018.00809
Nalls MA, Pankratz N, Lill CM et al (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46:989–993. https://doi.org/10.1038/ng.3043
Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140:1959–1976. https://doi.org/10.1093/brain/awx118
Moussaud S, Jones DR, Moussaud-Lamodière EL et al (2014) Alpha-synuclein and tau: teammates in neurodegeneration? Mol Neurodegener 9:43. https://doi.org/10.1186/1750-1326-9-43
Pan L, Li C, Meng L, et al (2022) Correspondence to : Zhentao Zhang SC SC
Osterhaus A, Groen J, Bildt M Van De, et al (1997) Ȋ-Synuclein in Lewy bodies endogenous proviruses as “ mementos ”? 839–840
Tampi RR, Young JJ, Tampi D (2019) Behavioral symptomatology and psychopharmacology of Lewy body dementia, 1st ed. Elsevier B.V.
Yang S, Chen W, Su C, Liu C (2018) Incidence and comorbidity of dementia with Lewy bodies : a population-based cohort study. 2018. https://doi.org/10.1155/2018/7631951
Walker Z, Possin KL, Boeve BF, Aarsland D (2015) Non-Alzheimer’s dementia 2 lewy body dementias. Lancet 386:1683–1697. https://doi.org/10.1016/S0140-6736(15)00462-6
Lozano CS, Tam J, Lozano AM (2017) The changing landscape of surgery for Parkinson ’ s disease 00:1–12. https://doi.org/10.1002/mds.27228
Outeiro TF, Koss DJ, Erskine D et al (2019) Dementia with Lewy bodies : an update and outlook 8:1–18
Schade S, Mollenhauer B (2014) Biomarkers in biological fluids for dementia with Lewy bodies. 1–7. https://doi.org/10.1186/s13195-014-0072-3
Van SI, Koel-simmelink MJA, Vergouw LJM et al (2020) Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies : a proteomic approach 2:1–15
Capouch SD, Farlow MR, Brosch JR (2018) A review of dementia with Lewy bodies’ impact, diagnostic criteria and treatment. Neurol Ther 7:249–263. https://doi.org/10.1007/s40120-018-0104-1
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150. https://doi.org/10.1038/nrneurol.2017.188
Huang Z, Wong LW, Su Y et al (2020) Blood-brain barrier integrity in the pathogenesis of Alzheimer’s disease. Front Neuroendocrinol 59:100857. https://doi.org/10.1016/j.yfrne.2020.100857
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27:9115–9129. https://doi.org/10.1523/JNEUROSCI.2361-07.2007
Liggins C, Snyder HM, Silverberg N et al (2014) International Alzheimer’s Disease Research Portfolio (IADRP) aims to capture global Alzheimer’s disease research funding. Alzheimer’s Dement 10:405–408. https://doi.org/10.1016/j.jalz.2013.12.013
Huang LK, Chao SP, Hu CJ (2020) Clinical trials of new drugs for Alzheimer disease. J Biomed Sci 27:1–13. https://doi.org/10.1186/s12929-019-0609-7
Sevigny J, Chiao P, Bussière T et al (2016) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537:50–56. https://doi.org/10.1038/nature19323
Pickett EK, Herrmann AG, Mcqueen J et al (2019) Amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s disease article amyloid beta and tau cooperate to cause reversible behavioral and transcriptional deficits in a model of Alzheimer’s D. Cell Rep 29:3592-3604.e5. https://doi.org/10.1016/j.celrep.2019.11.044
Sutaria DS, Badawi M, Phelps MA, Schmittgen TD (2017) Achieving the promise of therapeutic extracellular vesicles: the devil is in details of therapeutic loading. Pharm Res 34:1053–1066. https://doi.org/10.1007/s11095-017-2123-5
Tang Z, Ioja E, Bereczki E et al (2015) MTor mediates tau localization and secretion: implication for Alzheimer’s disease. Biochim Biophys Acta - Mol Cell Res 1853:1646–1657. https://doi.org/10.1016/j.bbamcr.2015.03.003
Watanabe R, Wei L, Huang J (2011) MTOR signaling, function, novel inhibitors, and therapeutic targets. J Nucl Med 52:497–500. https://doi.org/10.2967/jnumed.111.089623
Ozcelik S, Fraser G, Castets P et al (2013) Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS ONE 8:2–8. https://doi.org/10.1371/journal.pone.0062459
Bergmann L, Kube U, Doehn C et al (2015) Everolimus in metastatic renal cell carcinoma after failure of initial anti-VEGF therapy: final results of a noninterventional study. BMC Cancer 15:1–10. https://doi.org/10.1186/s12885-015-1309-7
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122:3589–3594. https://doi.org/10.1242/jcs.051011
Chen G, Huang AC, Zhang W, et al (2019) Associated with anti-PD-1 response. 560:382–386. https://doi.org/10.1038/s41586-018-0392-8.Exosomal
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318. https://doi.org/10.1038/nrm2672
Motoi Y, Shimada K, Ishiguro K, Hattori N (2014) Lithium and autophagy ACS Chem Neurosci 5:434–442. https://doi.org/10.1021/cn500056q
Park D, Jeong H, Lee MN et al (2016) Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci Rep 6:1–11. https://doi.org/10.1038/srep21772
Schaeffer V, Lavenir I, Ozcelik S et al (2012) Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain 135:2169–2177. https://doi.org/10.1093/brain/aws143
Lonskaya I, Hebron ML, Desforges NM et al (2013) Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med 5:1247–1262. https://doi.org/10.1002/emmm.201302771
Kim YD, Il JE, Nah J et al (2017) Pimozide reduces toxic forms of tau in TauC3 mice via 5′ adenosine monophosphate-activated protein kinase-mediated autophagy. J Neurochem 142:734–746. https://doi.org/10.1111/jnc.14109
Jose J, Valenzuela F, Varo RS, Castro CM (2020) Enhancing microtubule stabilization rescues cognitive deficits and ameliorates pathological phenotype in an amyloidogenic Alzheimer’s disease model. Sci Rep 1–17. https://doi.org/10.1038/s41598-020-71767-4
Ballatore C, Brunden KR, Huryn DM et al (2012) Microtubule stabilizing agents as potential treatment for Alzheimers disease and related neurodegenerative tauopathies. J Med Chem 55:8979–8996. https://doi.org/10.1021/jm301079z
Faustino C, Pinheiro L (2018) Therapeutic Strategies targeting tau protein: implications for Alzheimer’s disease. 1–16
Makani V, Zhang B, Han H et al (2016) Evaluation of the brain-penetrant microtubule-stabilizing agent, dictyostatin, in the PS19 tau transgenic mouse model of tauopathy. Acta Neuropathol Commun 4:1–12. https://doi.org/10.1186/s40478-016-0378-4
Harrington CR, Storey JMD, Clunas S et al (2015) Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer disease. J Biol Chem 290:10862–10875. https://doi.org/10.1074/jbc.M114.616029
Jadhav S, Avila J, Schöll M et al (2019) A walk through tau therapeutic strategies. Acta Neuropathol Commun 7:22. https://doi.org/10.1186/s40478-019-0664-z
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031. https://doi.org/10.1016/S0140-6736(10)61349-9
Pickhardt M, Neumann T, Schwizer D et al (2015) Identification of small molecule inhibitors of tau aggregation by targeting monomeric tau as a potential therapeutic approach for tauopathies. Curr Alzheimer Res 12:814–828. https://doi.org/10.2174/156720501209151019104951
Derisbourg M, Leghay C, Chiappetta G et al (2015) Role of the tau N-terminal region in microtubule stabilization revealed by new endogenous truncated forms. Sci Rep 5:1–10. https://doi.org/10.1038/srep09659
Liu K, Liu Y, Li L et al (2016) Glycation alter the process of tau phosphorylation to change Tau isoforms aggregation property. Biochim Biophys Acta - Mol Basis Dis 1862:192–201. https://doi.org/10.1016/j.bbadis.2015.12.002
Wilhelmus MMM, de Jager M, Bakker ENTP, Drukarch B (2014) Tissue transglutaminase in Alzheimer’s disease: involvement in pathogenesis and its potential as a therapeutic target. J Alzheimers Dis 42(Suppl 3):S289-303. https://doi.org/10.3233/JAD-132492
Neurochemistry JOF (2013) AstraZeneca R&D, Innovative medicines CNS and pain, S € odert € alje, Sweden. 446–456. https://doi.org/10.1111/jnc.12203
Mart L, Gonzalo-consuegra C, Marta G, et al (2021) Tideglusib, a non-ATP competitive inhibitor of GSK-3 β as a drug candidate for the treatment of amyotrophic lateral sclerosis
Malpas CB, Vivasha L, Genc S et al (2016) A phase iia randomized control trial of VEL015 (sodium selenate) in mild-moderate Alzheimer’s disease. J Alzheimer’s Dis 54:223–232. https://doi.org/10.3233/JAD-160544
Voronkov M, Braithwaite SP, Stock JB (2011) Phosphoprotein phosphatase 2A: a novel druggable target for Alzheimer’s disease. Future Med Chem 3:821–833. https://doi.org/10.4155/fmc.11.47
Theunis C, Crespo-Biel N, Gafner V, et al (2013) Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in Tau.P301L mice that model tauopathy. PLoS One 8. https://doi.org/10.1371/journal.pone.0072301
Davtyan H, Chen WW, Zagorski K et al (2017) MultiTEP platform-based DNA epitope vaccine targeting N-terminus of tau induces strong immune responses and reduces tau pathology in THY-Tau22 mice. Vaccine 35:2015–2024. https://doi.org/10.1016/j.vaccine.2017.03.020
Schroeder SK, Joly-amado A, Gordon MN, Morgan D (2015). Tau-directed immunotherapy : a promising strategy for treating Alzheimer’ s disease and other tauopathies. https://doi.org/10.1007/s11481-015-9637-6
Hardy J, Selkoe DJ (2013) The amyloid hypothesis of Alzheimer’s disease : progress and problems on the road to therapeutics. 353. https://doi.org/10.1126/science.1072994
Gilman S, Koller M, Black RS, et al (2005) Clinical effects of A  immunization (AN1792) in patients with AD in an interrupted trial
Krishnamurthy PK, Deng Y, Sigurdsson EM et al (2011) Mechanistic studies of antibody-mediated clearance of tau aggregates using an ex vivo brain slice model 2:1–6. https://doi.org/10.3389/fpsyt.2011.00059
Czerkowicz J, Chen W, Wang Q et al (2017) [P4–039]: Pan-tau antibody Biib076 exhibits promising safety and biomarker profile in cynomolgus monkey toxicity study. Alzheimer’s Dement 13:2017. https://doi.org/10.1016/j.jalz.2017.06.1903
Collin L, Bohrmann B, Göpfert U et al (2014) Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease. Brain 137:2834–2846. https://doi.org/10.1093/brain/awu213
Chai X, Wu S, Murray TK et al (2011) Passive immunization with anti-tau antibodies in two transgenic models 286:34457–34467. https://doi.org/10.1074/jbc.M111.229633
Chai X, Wu S, Murray TK et al (2011) Passive immunization with anti-tau antibodies in two transgenic models: reduction of tau pathology and delay of disease progression. J Biol Chem 286:34457–34467. https://doi.org/10.1074/jbc.M111.229633
Logovinsky V, Satlin A, Lai R et al (2016) Safety and tolerability of BAN2401 - a clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimer’s Res Ther 8:1–10. https://doi.org/10.1186/s13195-016-0181-2
Cummings JL, Cohen S, Van Dyck CH et al (2018) A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 90:E1889–E1897. https://doi.org/10.1212/WNL.0000000000005550
Yang T, Dang Y, Ostaszewski B et al (2019) Target engagement in an Alzheimer trial: crenezumab lowers amyloid β oligomers in cerebrospinal fluid. Ann Neurol 86:215–224. https://doi.org/10.1002/ana.25513
Tariot PN, Lopera F, Langbaum JB et al (2018) The Alzheimer’s prevention initiative autosomal-dominant Alzheimer’s disease trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s diseas. Alzheimer’s Dement Transl Res Clin Interv 4:150–160. https://doi.org/10.1016/j.trci.2018.02.002
Soeda Y, Takashima A (2020) New insights into drug discovery targeting tau protein 13:1–24. https://doi.org/10.3389/fnmol.2020.590896
Devos SL, Miller RL, Schoch KM, et al (2017) Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med 9:eaag0481
Bennett CF, Krainer AR, Cleveland DW (2019) Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci 42:385–406. https://doi.org/10.1146/annurev-neuro-070918-050501
Crooke ST, Baker BF, Kwoh TJ et al (2016) Integrated safety assessment of 2’-O-methoxyethyl chimeric antisense oligonucleotides in nonhuman primates and healthy human volunteers. Mol Ther 24:1771–1782. https://doi.org/10.1038/mt.2016.136
Ariyoshi J, Momokawa D, Eimori N et al (2015) Development of novel antisense oligonucleotides for the functional regulation of RNA-induced silencing complex (RISC) by promoting the release of microRNA from RISC. Bioconjug Chem 26:2454–2460. https://doi.org/10.1021/acs.bioconjchem.5b00501
Wurster CD, Ludolph AC (2018) Nusinersen for spinal muscular atrophy. Ther Adv Neurol Disord 11:1756285618754459
Brunet De Courssou JB, Durr A, Adams D et al (2022) Antisense therapies in neurological diseases. Brain 145:816–831. https://doi.org/10.1093/brain/awab423
Mummery CJ, Junge C, Kordasiewicz HB et al (2021) Results of the first-in-human, randomized, double-blind, placebo-controlled phase 1b study of lumbar intrathecal bolus administrations of antisense oligonucleotide (ISIS 814907; BIIB080) targeting tau mRNA in patients with mild Alzheimer’s disease. Alzheimer’s Dement 17:2–3. https://doi.org/10.1002/alz.051871
Schneider LS, Thomas RG, Hendrix S et al (2019) Safety and efficacy of edonerpic maleate for patients with mild to moderate Alzheimer disease: a phase 2 randomized clinical trial. JAMA Neurol 76:1330–1339
Fukushima T, Nakamura A, Iwakami N et al (2011) T-817MA, a neuroprotective agent, attenuates the motor and cognitive impairments associated with neuronal degeneration in P301L tau transgenic mice. Biochem Biophys Res Commun 407:730–734. https://doi.org/10.1016/j.bbrc.2011.03.091
Devanand DP, Andrews H, Kreisl WC, et al (2020) Antiviral therapy : valacyclovir treatment of Alzheimer’s disease (VALAD) trial : protocol for a controlled, treatment trial. 1:1–10. https://doi.org/10.1136/bmjopen-2019-032112
Wozniak MA, Frost AL, Preston CM, Itzhaki RF (2011) Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1. 6. https://doi.org/10.1371/journal.pone.0025152
Wang X, Sun G, Feng T et al (2019) Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29:787–803. https://doi.org/10.1038/s41422-019-0216-x
Herrmann N, Ruthirakuhan M, Gallagher D et al (2019) Randomized placebo-controlled trial of nabilone for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry 27:1161–1173. https://doi.org/10.1016/j.jagp.2019.05.002
Grossberg GT, Kohegyi E, Mergel V et al (2020) Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. Am J Geriatr Psychiatry 28:383–400. https://doi.org/10.1016/j.jagp.2019.09.009
Reddy AP, Yin X, Sawant N, Reddy PH (2021) Protective effects of antidepressant citalopram against abnormal APP processing and amyloid beta-induced mitochondrial dynamics, biogenesis, mitophagy and synaptic toxicities in Alzheimer’s disease. Hum Mol Genet 30:847–864. https://doi.org/10.1093/hmg/ddab054
West T, Hu Y, Verghese PB, et al (2017) Preclinical and clinical development of ABBV-8E12, a humanized anti-tau antibody, for treatment of Alzheimer’s disease and other tauopathies. J Prev Alzheimer’s Dis 4:236–241. https://doi.org/10.14283/jpad.2017.36
Sabbagh MN, Decourt B (2022) COR388 (atuzaginstat): an investigational gingipain inhibitor for the treatment of Alzheimer disease. Expert Opin Investig Drugs 31:987–993. https://doi.org/10.1080/13543784.2022.2117605
Wang HY, Bakshi K, Frankfurt M et al (2012) Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A. J Neurosci 32:9773–9784. https://doi.org/10.1523/JNEUROSCI.0354-12.2012
Wang Y-J, Ren Q-G, Gong W-G, et al (2016) Escitalopram attenuates β-amyloid-induced tau hyperphosphorylation in primary hippocampal neurons through the 5-HT1A receptor mediated Akt/GSK-3β pathway. Oncotarget 7:13328–13339. https://doi.org/10.18632/oncotarget.7798
Sansone RA, Sansone LA (2014) Serotonin norepinephrine reuptake inhibitors: a pharmacological comparison. Innov Clin Neurosci 11:37–42
Shukla S, Tekwani BL (2020) Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front Pharmacol 11:1–20. https://doi.org/10.3389/fphar.2020.00537
Kuo Y-M, Nwankwo EI, Nussbaum RL et al (2019) Translational inhibition of α-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice. Am J Neurodegener Dis 8:1–15
Gai A, Seung-Hye L, Oskar A, et al (2021) Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer’s disease. Sci Transl Med 13:eabb2639. https://doi.org/10.1126/scitranslmed.abb2639
Wagner J, Krauss S, Shi S et al (2015) Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies. Acta Neuropathol 130:619–631. https://doi.org/10.1007/s00401-015-1483-3
Niu X, Chen J, Gao J (2019) Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci 14:480–496. https://doi.org/10.1016/j.ajps.2018.09.005
Alajangi HK, Kaur M, Sharma A, et al (2022) Blood–brain barrier : emerging trends on transport models and new ‑ age strategies for therapeutics intervention against neurological disorders. Mol Brain 1–28. https://doi.org/10.1186/s13041-022-00937-4
He Z, Guo JL, Mcbride JD et al (2018) Amyloid- β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Publ Gr 24:29–38. https://doi.org/10.1038/nm.4443
Sonawane SK, Ahmad A, Chinnathambi S (2019). Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. https://doi.org/10.1021/acsomega.9b01411
Saraiva C, Praça C, Ferreira R et al (2016) Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 235:34–47. https://doi.org/10.1016/j.jconrel.2016.05.044
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Ealias AM, Saravanakumar MP (2017) A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf Ser Mater Sci Eng 263. https://doi.org/10.1088/1757-899X/263/3/032019
Jeevanandam J, Barhoum A, Chan YS et al (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 9:1050–1074. https://doi.org/10.3762/bjnano.9.98
Patra JK, Das G, Fraceto LF et al (2018) Nano based drug delivery systems: recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J Nanobiotechnology 16:1–33. https://doi.org/10.1186/s12951-018-0392-8
Fuller MA, Köper I (2019) Biomedical applications of polyelectrolyte coated spherical gold nanoparticles. Nano Converg 6. https://doi.org/10.1186/s40580-019-0183-4
Bhattacharjee S (2019) Polymeric nanoparticles. Princ Nanomedicine 195–240. https://doi.org/10.1201/9780429031236-8
Kango S, Kalia S, Celli A et al (2013) Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites - a review. Prog Polym Sci 38:1232–1261. https://doi.org/10.1016/j.progpolymsci.2013.02.003
Parveen S, Misra R, Sahoo SK (2012) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine Nanotechnology, Biol Med 8:147–166. https://doi.org/10.1016/j.nano.2011.05.016
Ceña V, Játiva P (2018) Nanoparticle crossing of blood-brain barrier: a road to new therapeutic approaches to central nervous system diseases. Nanomedicine 13:1513–1516. https://doi.org/10.2217/nnm-2018-0139
Li X, Tsibouklis J, Weng T et al (2017) Nano carriers for drug transport across the blood–brain barrier. J Drug Target 25:17–28. https://doi.org/10.1080/1061186X.2016.1184272
Petros RA, Desimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9:615–627. https://doi.org/10.1038/nrd2591
Fan Y, Marioli M, Zhang K (2021) Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal 192:113642. https://doi.org/10.1016/j.jpba.2020.113642
Mishra V, Bansal KK, Verma A et al (2018) Solid lipid nanoparticles: emerging colloidal nano drug delivery systems. Pharmaceutics 10:1–21. https://doi.org/10.3390/pharmaceutics10040191
Bernkop-Schnürch A, Müllertz A, Rades T (2019) Self-emulsifying drug delivery systems (SEDDS) – The splendid comeback of an old technology. Adv Drug Deliv Rev 142:1–2. https://doi.org/10.1016/j.addr.2019.08.002
L. Shinde R, B. Jindal A, V. Devarajan P, (2011) Microemulsions and nanoemulsions for targeted drug delivery to the brain. Curr Nanosci 7:119–133. https://doi.org/10.2174/157341311794480282
Rehman FU, Shah KU, Shah SU et al (2017) From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin Drug Deliv 14:1325–1340. https://doi.org/10.1080/17425247.2016.1218462
Huang D, Wu D (2018) Biodegradable dendrimers for drug delivery. Mater Sci Eng C 90:713–727. https://doi.org/10.1016/j.msec.2018.03.002
Vega-Vásquez P, Mosier NS, Irudayaraj J (2020) Nanoscale drug delivery systems: from medicine to agriculture. Front Bioeng Biotechnol 8:1–16. https://doi.org/10.3389/fbioe.2020.00079
Chauhan AS (2018) Dendrimers for drug delivery. Molecules 23. https://doi.org/10.3390/molecules23040938
Kulhari H, Pooja D, Singh MK, Chauhan AS (2015) Optimization of carboxylate-terminated poly(amidoamine) dendrimer-mediated cisplatin formulation. Drug Dev Ind Pharm 41:232–238. https://doi.org/10.3109/03639045.2013.858735
Wilson B, Kumar M, Santhi K, et al (2008) Poly (n -butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. 0:1–10. https://doi.org/10.1016/j.brainres.2008.01.039
Fornaguera C, Feiner-Gracia N, Calderó G et al (2015) Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale 7:12076–12084. https://doi.org/10.1039/c5nr03474d
Lungare S, Hallam K, Badhan RKS (2016) Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int J Pharm. https://doi.org/10.1016/j.ijpharm.2016.09.042
López ES, Ettcheto M, Egea MA, et al (2018) Memantine loaded PLGA PEGylated nanoparticles for Alzheimer ’ s disease : in vitro and in vivo characterization. J Nanobiotechnology 1–16. https://doi.org/10.1186/s12951-018-0356-z
Fazil M, Haque S, Kumar M, Baboota S (2012) European Journal of Pharmaceutical Sciences Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci 47:6–15. https://doi.org/10.1016/j.ejps.2012.04.013
Wilson B, Samanta MK, Santhi K et al (2010) Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine Nanotechnology, Biol Med 6:144–152. https://doi.org/10.1016/j.nano.2009.04.001
Mourtas S, Canovi M, Zona C et al (2011) Biomaterials curcumin-decorated nanoliposomes with very high affinity for amyloid- b 1–42 peptide. Biomaterials 32:1635–1645. https://doi.org/10.1016/j.biomaterials.2010.10.027
Huo X, Zhang Y, Jin X, et al (2018) PT. J Photochem Photobiol B Biol #pagerange#. https://doi.org/10.1016/j.jphotobiol.2018.11.008
Sachdeva A, Misra S, Kaur IP, Chopra K (2014) Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive de fi cits : behavioral and biochemical evidence. Eur J Pharmacol 1–9. https://doi.org/10.1016/j.ejphar.2014.11.014
Matteis L De, Martín-rapún R, De JM (2018) Nanotechnology in personalized medicine : a promising tool for Alzheimer’s disease treatment. 4602–4615. https://doi.org/10.2174/0929867324666171012112026.The
Cummings J (2019) The role of biomarkers in Alzheimer’s disease drug development. Adv Exp Med Biol 1118:29–61. https://doi.org/10.1007/978-3-030-05542-4_2
Schöll M, Maass A, Mattsson N et al (2019) Biomarkers for tau pathology. Mol Cell Neurosci 97:18–33. https://doi.org/10.1016/j.mcn.2018.12.001
Blennow K, Hampel H, Weiner M, Zetterberg H (2010) Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 6:131–144. https://doi.org/10.1038/nrneurol.2010.4
Zetterberg H, Bendlin BB (2021) Biomarkers for Alzheimer’s disease—preparing for a new era of disease-modifying therapies. Mol Psychiatry 26:296–308. https://doi.org/10.1038/s41380-020-0721-9
Kolarova M, García-Sierra F, Bartos A, et al (2012) Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis 2012. https://doi.org/10.1155/2012/731526
Eusebi P, Giannandrea D, Biscetti L et al (2017) Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 32:1389–1400. https://doi.org/10.1002/mds.27110
Constantinides VC, Majbour NK, Paraskevas GP et al (2021) Cerebrospinal fluid α-synuclein species in cognitive and movements disorders. Brain Sci 11:1–12. https://doi.org/10.3390/brainsci11010119
Balasa R, Bianca C, Septimiu V et al (2018) The matrix metalloproteinases panel in multiple sclerosis patients treated with natalizumab: a possible answer to natalizumab non-responders. CNS Neurol Disord - Drug Targets 17:464–472
Nordengen K, Kirsebom BE, Henjum K et al (2019) Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflammation 16. https://doi.org/10.1186/s12974-019-1399-2
Podlesniy P, Silva J, Lladó A, et al (2013) Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol 74. https://doi.org/10.1002/ana.23955
Guo L-H, Alexopoulos P, Wagenpfeil S et al (2013) Plasma proteomics for the identification of Alzheimer disease. Alzheimer Dis Assoc Disord 27:337–342. https://doi.org/10.1097/WAD.0b013e31827b60d2
Fossati S, Ramos Cejudo J, Debure L et al (2019) Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer’s disease. Alzheimer’s Dement Diagnosis, Assess Dis Monit 11:483–492. https://doi.org/10.1016/j.dadm.2019.05.001
Khalil M, Teunissen CE, Otto M et al (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14:577–589. https://doi.org/10.1038/s41582-018-0058-z
Kester MI, Teunissen CE, Crimmins DL et al (2015) Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 72:1275–1280. https://doi.org/10.1001/jamaneurol.2015.1867
Sjödin S, Andersson KKA, Mercken M et al (2015) APLP1 as a cerebrospinal fluid biomarker for γ-secretase modulator treatment. Alzheimer’s Res Ther 7:1–10. https://doi.org/10.1186/s13195-015-0160-z
Begcevic I, Brinc D, Brown M et al (2018) Brain-related proteins as potential CSF biomarkers of Alzheimer’s disease: a targeted mass spectrometry approach. J Proteomics 182:12–20. https://doi.org/10.1016/j.jprot.2018.04.027
Bjorkli C, Sandvig A, Sandvig I (2020) Bridging the gap between fluid biomarkers for Alzheimer’s disease, model systems, and patients. Front Aging Neurosci 12:272. https://doi.org/10.3389/fnagi.2020.00272
Yuan A, Rao M V., Veeranna, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9. https://doi.org/10.1101/cshperspect.a018309
Cicognola C, Brinkmalm G, Wahlgren J et al (2019) Novel tau fragments in cerebrospinal fluid: relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol 137:279–296. https://doi.org/10.1007/s00401-018-1948-2
Blennow K (2017) A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol Ther 6:15–24. https://doi.org/10.1007/s40120-017-0073-9
Zetterberg H (2015) Cerebrospinal fluid biomarkers for Alzheimer’s disease: current limitations and recent developments. Curr Opin Psychiatry 28:402–409. https://doi.org/10.1097/YCO.0000000000000179
Bateman RJ, Blennow K, Doody R, et al (2019) Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD task force report. J Prev Alzheimer’s Dis 6:169–173. https://doi.org/10.14283/jpad.2019.21
Palmqvist S (2015) Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. 0:
Agrawal M, Biswas A, Levy CE (2015) Molecular diagnostics of neurodegenerative disorders 2:1–10. https://doi.org/10.3389/fmolb.2015.00054
Liu C-C, Liu C-C, Kanekiyo T et al (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9:106–118
Pires G, McElligott S, Drusinsky S et al (2019) Secernin-1 is a novel phosphorylated tau binding protein that accumulates in Alzheimer’s disease and not in other tauopathies. Acta Neuropathol Commun 7:1–17. https://doi.org/10.1186/s40478-019-0848-6
Fonseca-santos B (2015) Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. 4981–5003
Poudel P, Park S (2022) Recent advances in the treatment of Alzheimer’s disease using nanoparticle-based drug delivery systems
Acknowledgements
We would like to thank Dr. Alisha Jones (Incoming Assistant Professor at NYU) for critically reading the manuscript. The authors gratefully acknowledge RPBlab and GSlab members for critical suggestions.
Funding
Our lab is supported by SERB, DBT and ICMR, Government of India, grants which are duly acknowledged. AK, HKA and AS are supported by UGC-JRF, DHR-YSS and ICMR-SRF fellowships, respectively.
Author information
Authors and Affiliations
Contributions
GS and RPB conceived the plan. PK, AK, and HKA wrote first draft. AS, PKJ, GS, and RPB edited and finalized the draft in coordination with other authors. All authors have approved it for publication.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Neurological disorders are increasingly associated with fatalities globally.
• Tauopathies are mostly caused by hyperphosphorylation of tau protein.
• Drugs under development target pathways like mTOR, phosphorylation, and aggregation.
• Biomarkers for an early diagnosis like phosphorylated sites are in developmental stages.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, P., Khera, A., Alajangi, H.K. et al. Role of Tau in Various Tauopathies, Treatment Approaches, and Emerging Role of Nanotechnology in Neurodegenerative Disorders. Mol Neurobiol 60, 1690–1720 (2023). https://doi.org/10.1007/s12035-022-03164-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03164-z