Abstract
Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) is an autosomal dominant neurodegenerative disorder, which has three cardinal features: behavioral and personality changes, cognitive impairment, and motor symptoms. FTDP-17 was defined during the International Consensus Conference in Ann Arbor, Michigan, in 1996. The prevalence and incidence remain unknown but FTDP-17 is an extremely rare condition. It is caused by mutations in the tau gene, which encodes a microtubule-binding protein. Over 100 families with 38 different mutations in the tau gene have been identified worldwide. The phenotype of FTDP-17 varies not only between families carrying different mutations but also between and within families carrying the same mutations. The pathogenetic mechanisms underlying the disorder are thought to be related to the altered proportion of tau isoforms or to the ability of tau to bind microtubules and to promote microtubule assembly. Definitive diagnosis of FTDP-17 requires a combination of characteristic clinical and pathological features and molecular genetic analysis. Genetic counseling should be offered to affected and at-risk individuals; for most subtypes, penetrance is incomplete. Currently, treatment for FTDP-17 is only symptomatic and supportive. The prognosis and rate of the disease's progression vary considerably among individual patients and genetic kindreds, ranging from life expectancies of several months to several years, and, in exceptional cases, as long as two decades.
Similar content being viewed by others
Disease name and synonyms
Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).
Definition/diagnostic criteria
The term frontotemporal dementia and parkinsonism linked to chromosome 17 was defined during the International Consensus Conference in Ann Arbor, Michigan, in 1996 [1]. At the time, affected individuals with frontotemporal dementia and parkinsonism linked to the wld locus on chromosome 17 had been identified within 13 families. This syndrome is a familial disorder with autosomal dominant inheritance. The three major clinical features include behavioral disturbances, cognitive impairment, and parkinsonism. There are no strict diagnostic criteria for FTDP-17. Nevertheless, FTDP-17 should be considered in the differential diagnosis in the presence of one or more of the following [2]:
• Age of onset of neurological symptoms between the third and fifth decades;
• Progressive neuropsychiatric syndrome including personality and behavioral abnormalities and/or frontotemporal dementia;
• Parkinsonism-plus syndrome (bradykinesia, rigidity, postural instability, paucity of resting tremor, and poor or no response to dopaminergic therapy) frequently associated with falls and supranuclear gaze palsy and less commonly associated with apraxia, dystonia, and lateralization;
• Progressive speech difficulties from the onset of the illness;
• Seizure disorder poorly controlled by standard anticonvulsant therapy;
• Positive family history suggestive of autosomal dominant inheritance of a neurodegenerative disorder, even if there has been variability in clinical presentations.
Epidemiology
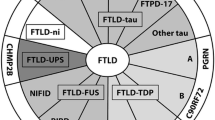
FTDP-17 is an extremely rare condition. Its prevalence and incidence remain unknown. Over 100 families with FTDP-17 have been reported to date in numerous countries (USA, Great Britain, Japan, Netherlands, France, Canada, Australia, Italy, Germany, Israel, Ireland, Spain and Sweden). Some of these families share a common founder [3]. We estimate that there have been about 50–600 patients described, with fewer than 70 individuals still living. Molecular genetic studies have identified 38 unique mutations in these families (Figure 1). The most common mutations, accounting for approximately 60% of known cases, are P301L, N279K and a splice site mutation (exon 10 +16) [4].
Etiology
Mutations in the tau gene account for the vast majority of FTDP-17 cases. Tau is a microtubule-binding protein abundant in neurons and glia. In neurons, it is predominantly expressed in axons. Tau binds to and stabilizes microtubules and promotes microtubule assembly. The majority of the currently known mutations in coding region occur within the microtubule-binding region of tau gene. Most known mutations in non-coding regions affect the splicing of exon 10 [4]. The pathogenetic mechanisms in FTDP-17 are thought to be related to the altered proportion of tau isoforms or to the ability of tau to bind microtubules and to promote microtubule assembly.
Mutations in tau gene associated with FTDP-17 fall into two broad mechanistic groups. One group contains coding mutations (missense and two deletions) that, in recombinant protein studies and in transfected cell assays, have been shown to disrupt the binding of tau to microtubules. In addition, the majority of these mutations have also been shown to accelerate the aggregation of recombinant tau in the presence of polyanions. Thus, overall, these mutations are predicted both to increase the proportion of tau that is unbound to microtubules and available for aggregation and also to increase directly the tendency of the unbound tau to form filaments. The second group of tau mutations appears to cause disease by disrupting the alternative splicing of exon 10 and hence the ratio of 4R: 3R tau. These mutations comprise a mixture of coding changes, within exon 10 (N279K, delK280, L284L, N296N/H, delN296, P301L/S, G303V, and S305S/N) and also intronic mutations close to the 5' splice site of exon 10 (at positions +3, +11, +12, +13, +14, +16, +19, and +29). All but three of these mutations have been demonstrated to increase the splicing-in of exon 10 and hence the proportion of 4R tau. The exceptions are delK280, +19, and, +29 mutations that show increased 3R: 4R ratio as compared with normal condition [5]. The +29 mutation has been detected in both affected individuals with the FTD phenotype and normal controls [6–8]. Therefore, it is still unclear whether this splicing mutation contributes to disease development.
Families with FTD linked to chromosome 17q21 without tau mutations
Six FTDP-17 kindreds have been identified in which neither a tau mutation nor tau pathology has been detected, despite genetic linkage to the same region of chromosome 17q21 that contains the tau gene [7–12]. Their clinical phenotype is indistinguishable from that of cases with known tau mutations. The specific mutations and their clinical presentations are summarized in Table 1. Studies of this group of individuals provide valuable information for elucidating the etiology of FTDP-17, as it introduces the possibility that further mutations in the introns or regulatory regions of tau, or other causative genes near the tau locus may yet be discovered. For example, mutations in the intronic sequence adjacent to the stem loop structure in exon 10 have been identified that alter tau splicing to increase soluble 3R tau, leading to increased tau proteolysis and neuronal apoptosis without deposition of insoluble tau aggregates [13].
Extensive ubiquitin-positive, tau-negative, cytoplasmic and intranuclear neuronal inclusions have been observed in layer II of the cerebral cortex and in the dentate gyrus of the hippocampus in three of the kindreds lacking a tau mutation [14, 15]. The intranuclear inclusions are unlikely to result from a trinucleotide repeat expansion mutation in that they failed to stain when exposed to an antibody that recognizes proteins with polyglutamine tracts [7]. Descriptions of similar intranuclear inclusions in patients with FTD and motor neuron disease [14, 15] suggest that they may turn out to be specific for chromosome 17-linked FTD without tau mutations.
Clinical description
Symptoms and signs
The onset of FTDP-17 is usually insidious. Affected individuals who have reached the fully developed stage of the disease present with a constellation of signs including at least two of the three cardinal features of FTDP-17.
• Behavioral and personality disturbances;
• Cognitive deficits;
• Motor dysfunction (typically signs of parkinsonism-plus syndrome).
Clinical features of FTDP-17 vary considerably among affected individuals, regardless of whether they inherit the same or different mutations. Even members within a family, for example, can vary in their clinical presentation. The specific mutations and their characteristic clinical presentations are summarized in Table 2.
The behavioral and personality abnormalities can include disinhibition, apathy, defective judgment, compulsive behavior, hyper-religiosity, neglect of personal hygiene, alcoholism, illicit drug addiction, verbal and physical aggressiveness, family abuse, and other manifestations. While cognitive disturbances occur, memory, orientation, and visuospatial function are relatively preserved during early stages of the disease. Progressive speech difficulties with non-fluent aphasia and disorders of executive functions can be seen initially. Subsequently, memory, orientation, and visuospatial functions progressively deteriorate, while echolalia, palilalia, verbal and vocal perseverations develop. Finally, progressive dementia and mutism occur. Motor signs are also prominent. Parkinsonism can be the first manifestation of the disease, and in this regard it is important to note that some FTDP-17 patients were initially misdiagnosed as having Parkinson's disease or sporadic progressive supranuclear palsy. In some families, however, the parkinsonism occurs late in the course of the illness or not at all. The parkinsonism in FTDP-17 is characterized by rather symmetrical bradykinesia, postural instability, rigidity affecting equally axial and appendicular musculature, (usually) absence of resting tremor, and poor or no responsiveness to levodopa therapy. Other motor disturbances seen in FTDP-17 include dystonia unrelated to medications, supranuclear gaze palsy, upper and lower motor neuron dysfunction, myoclonus, postural and action tremors, eyelid opening and closing apraxia, dysphagia, and dysarthria.
Phenotype-genotype correlations
It is still very difficult to perform precise phenotype/genotype correlations in FTDP-17 since the clinical information available for some families is not detailed enough in or not accessible at all. Nevertheless, some patterns have emerged. The families with FTDP-17 fall into two major groups [16]:
• dementia predominant phenotype;
• parkinsonism-plus predominant phenotype.
The dementia predominant phenotype is more common and is usually seen in families with mutations in exons 1, 9, 11, 12, 13 and in exon 10.
The parkinsonism-plus predominant phenotype is usually seen in families with intronic and exonic mutations affecting exon 10 and leading to the selective overproduction of 4R tau isoforms. These categorizations should be viewed cautiously until more clinical and pathologic data become available.
Diagnostic methods
Characteristic clinical and pathological features of FTDP-17 coupled with a molecular genetic analysis of the tau gene are essential steps for a diagnosis.
Imaging studies such as computerized tomography (CT) and magnetic resonance imaging (MRI) can assist in establishing a diagnosis, mainly by excluding other diagnostic possibilities such as the presence of a brain tumor, abscess, multi-infarct state, or hydrocephalus.
Imaging studies
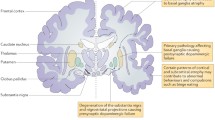
CT and MRI of the head usually demonstrate some dilation of the ventricular system and frontal, temporal, and parietal cortical atrophy (Figure 2A) [17–22]. MRI T2-weighted images may show accumulation of paramagnetic substances (iron) in mesencephalic nuclei [19]. In some kindreds, asymmetrical cortical atrophy is present. Functional imaging studies such as single photon emission computerized tomography (SPECT) and positron emission tomography (PET) also demonstrate significant abnormalities. PET with 2-deoxy-2-fluoro- [18F]-D-glucose (FDG) usually shows reduced frontal-parietal-temporal uptake patterns similar to those seen in sporadic frontotemporal dementia (FTD). PET with [18F]-fluoro-L-dopa (6FD) and [11C]-raclopride tracers reveals uptake abnormalities distinct from those seen in PD, in which the putamen is affected more than the caudate nucleus [23].
A) Head MRI (left panels) demonstrates cortical atrophy in frontal and temporal lobes. Positron emission tomography (PET) with 2-deoxy-2-fluoro- [18F]-D-glucose (FDG) PET (right panels) demonstrates hypometabolism in the same cortical regions. Middle panels show co-registration of MRI and FDG PET studies. B) Thermoregulatory sweating tests in the same patient with PPND. Shaded areas represent sweating over the anterior body surface. Distal anhidrosis is seen.
Laboratory findings
The routine serum, urine, cerebrospinal fluid and other body fluid studies are usually negative. Electroencephalography (EEG) findings are normal early in the disease process and progress to diffuse slowing as the disease advances, although slowing can sometimes be seen earlier in the course of the FTDP-17 illness [24]. In sporadic FTD, the slowing of background rhythms usually occurs late in the course of the illness. In individuals with the P301S mutation, EEG demonstrates sharp waves, spikes and epileptiform discharges [25]. Nerve conduction studies are normal. Electromyography may show neurogenic patterns related to lower motor neuron dysfunction [26]. Evoked potential studies are usually normal. Autonomic testing may show sudomotor impairment but not orthostatic hypotension (Figure 2B) [27]. There is very little information available on sleep studies in FTDP-17 kindreds. In a clinicopathological study of an affected patient with G389R mutation, a relationship between the reduced delta sleep and the decreased cortical and subcortical connectivity was reported [28]. Neuropsychological evaluation is of paramount significance in determining the severity and extent of cognitive and behavioral dysfunction in this disorder.
Differential diagnosis
Clinically, FTDP-17 may mimic several neurodegenerative diseases. The differential diagnosis of FTDP-17 includes disorders with initial signs such as behavioral and personality abnormalities, parkinsonism, lower motor neuron dysfunction or cognitive impairments. In the absence of a positive family history or molecular genetic data, FTDP-17 is most frequently confused with Pick's disease, sporadic progressive supranuclear palsy (PSP) or corticobasal degeneration (CBD). Other familial frontotemporal dementias, parkinson-plus syndromes, dementia with Lewy bodies (DLB), Parkinson's disease (PD), multiple system atrophy (MSA) should be excluded. A neuropathologic examination coupled with molecular genetic analysis of the tau gene are essential steps towards distinguishing FTDP-17 from other neurodegenerative diseases associated with tau deposition. The pathologic analysis should include immunohistochemical studies using multiple anti-tau antibodies.
Genetic counseling
Affected individuals should be counseled regarding the estimated probability of passing the genetic bases for their illness on to their offspring. As FTDP-17 is an autosomal dominantly inherited condition, each offspring of an affected individual will carry a 50% risk of inheriting the abnormal gene. Some of these mutations can be detected through genetic testing. The individual who inherits a mutation will not necessarily develop the same clinical syndrome as the parent, because penetrance may be incomplete, neurologic manifestations vary greatly even within families, or the individual might die from unrelated causes. In the case of the pallido-ponto-nigral degeneration (PPND) subtype of FTDP-17, however, penetrance is complete and virtually all individuals who inherit the mutation will become symptomatic during middle age.
At-risk individuals may sometimes choose to adopt rather than bear their own children. When faced with the option of genetic testing, some people prefer the greater certainty that a genetic diagnosis may afford when planning life decisions, while others find not knowing to be a lesser emotional burden as well as a lesser risk of being denied healthcare should their insurance carrier learn the test result. In either case the patient's choice should be respected.
Inquiring into the family history can also identify relatives who may be at risk of having inherited the genetic basis for FTDP-17 and who thus may be presymptomatic.
Clinical genetic testing of affected and presymptomatic individuals is commercially available for some mutations. However, there tends to be little interest among at-risk family members to undergo clinical genetic testing [29].
Antenatal diagnosis
Once a genetic basis for a subtype of FTDP-17 is discovered and testing for that gene becomes commercially available, it becomes possible, in principle, to identify that gene antenatally by sampling amniotic fluid or by preimplantation genetic diagnosis. Genetic counseling should accompany antenatal genetic diagnosis.
Management including treatment
Currently, a curative treatment for FTDP-17 does not exist, and most patients respond poorly, if at all, to levodopa. Palliative and symptomatic interventions are the mainstay of treatment. Physical therapy can be helpful to preserve some measure of mobility and independence in activities of daily living and to reduce the risk of falling. Stool softening agents are recommended for constipation. Speech therapy, swallowing assessment, and switching to a softer diet may be helpful for dysphagia. Anticholinergics may be helpful for sialorrhea or urinary frequency but carry the risk of aggravating mental confusion. Clozapine or quetiapine may be helpful for psychosis not explained by systemic infection or responsive to medication withdrawal. Antidepressant medications may be indicated for concurrent depression. Bed-bound patients will require frequent repositioning or air flow mattresses. Patients should be encouraged to discuss their treatment preferences with their families and their physicians and to indicate their choices by advance directives.
Prognosis
The prognosis varies considerably. Most patients with FTDP-17 will experience several months to one or two years of moderate impairment followed by an unremitting downward course of physical and cognitive decline. The rate of progression varies greatly among individual patients and genetic kindreds, ranging from life expectancies of several months to several years, and, in exceptional cases, as long as two decades. In addition to the clinical features described above, in the late stages of illness these patients often develop secondary medical problems associated with immobility including falling injuries and respiratory and urinary tract infections.
Unresolved questions
It is hoped that further research will identify the genetic modifiers and environmental factors that influence the variable penetrance and clinical expression of FTDP-17. The development of transgenic mice may provide an opportunity to test novel therapeutic agents in the near future.
References
Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D'Amato CJ, Gilman S: Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference Participants. Ann Neurol. 1997, 41: 706-715. 10.1002/ana.410410606.
Wszolek ZK, Tsuboi Y, Farrer M, Uitti RJ, Hutton ML: Hereditary tauopathies and parkinsonism. Adv Neurol. 2003, 91: 153-163.
Tsuboi Y, Baker M, Hutton ML, Uitti RJ, Rascol O, Delisle MB, Soulages X, Murrell JR, Ghetti B, Yasuda M, Komure O, Kuno S, Arima K, Sunohara N, Kobayashi T, Mizuno Y, Wszolek ZK: Clinical and genetic studies of families with the tau N279K mutation (FTDP-17). Neurology. 2002, 59: 1791-1793.
Ghetti B, Hutton M, Wszolek ZK: Frontotemporal dementia and parkinsonism linked to chromosome 17 associated with tau gene mutations (FTDP-17T). Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Edited by: Dickson D. Basel: ISN Neuropath Press 2003.
Wszolek ZK, Slowinski J, Golan M, Dickson DW: Frontotemporal dementia and parkinsonism linked to chromosome 17. Folia Neuropathol. 2005, 43: 258-270.
D'Souza I, Poorkaj P, Hong M, Nochlin D, Lee VM, Bird TD, Schellenberg GD: Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci U S A. 1999, 96: 5598-5603. 10.1073/pnas.96.10.5598.
Froelich Fabre S, Axelman P, Almkvist A, Basun H, Lannfelt L: Extended investigation of tau and mutation screening of other candidate genes on chromosome 17q21 in a Swedish FTDP-17 family. Am J Med Genet B Neuropsychiatr Genet. 2003, 121: 112-118. 10.1002/ajmg.b.20067.
Rosso SM, Kamphorst W, de Graaf B, Willemsen R, Ravid R, Niermeijer MF, Spillantini MG, Heutink P, van Swieten JC: Familial frontotemporal dementia with ubiquitin-positive inclusions is linked to chromosome 17q21-22. Brain. 2001, 124: 1948-1957. 10.1093/brain/124.10.1948.
Lendon CL, Lynch T, Norton J, McKeel DW, Busfield F, Craddock N, Chakraverty S, Gopalakrishnan G, Shears SD, Grimmett W, Wilhelmsen KC, Hansen L, Morris JC, Goate AM: Hereditary dysphasic disinhibition dementia: a frontotemporal dementia linked to 17q21-22. Neurology. 1998, 50: 1546-1555.
Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van den Broeck M, Backhovens H, van Swieten J, van Duijn CM, Van Broeckhoven C: Tau negative frontal lobe dementia at 17q21: significant finemapping of the candidate region to a 4.8 cM interval. Mol Psychiatry. 2002, 7: 1064-1074. 10.1038/sj.mp.4001198.
Kertesz A, Kawarai T, Rogaeva E, St George-Hyslop P, Poorkaj P, Bird TD, Munoz DG: Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology. 2000, 54: 818-827.
Bird TD, Wijsman EM, Nochlin D, Leehey M, Sumi SM, Payami H, Poorkaj P, Nemens E, Rafkind M, Schellenberg GD: Chromosome 17 and hereditary dementia: linkage studies in three non-Alzheimer families and kindreds with late-onset FAD. Neurology. 1997, 48: 949-954.
Stanford PM, Shepherd CE, Halliday GM, Brooks WS, Schofield PW, Brodaty H, Martins RN, Kwok JB, Schofield PR: Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain. 2003, 126: 814-826. 10.1093/brain/awg090.
Woulfe J, Kertesz A, Munoz DG: Frontotemporal dementia with ubiquitinated cytoplasmic and intranuclear inclusions. Acta Neuropathol (Berl). 2001, 102: 94-102.
Mackenzie IR, Feldman H: The relationship between extramotor ubiquitin-immunoreactive neuronal inclusions and dementia in motor neuron disease. Acta Neuropathol (Berl). 2003, 105: 98-102.
Reed LA, Wszolek ZK, Hutton M: Phenotypic correlations in FTDP-17. Neurobiol Aging. 2001, 22: 89-107. 10.1016/S0197-4580(00)00202-5.
Bird TD, Nochlin D, Poorkaj P, Cherrier M, Kaye J, Payami H, Peskind E, Lampe TH, Nemens E, Boyer PJ, Schellenberg GD: A clinical pathological comparison of three families with frontotemporal dementia and identical mutations in the tau gene (P301L). Brain. 1999, 122: 741-756. 10.1093/brain/122.4.741.
Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B: Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol. 1999, 58: 667-677.
Cordes M, Wszolek ZK, Calne DB, Rodnitzky RL, Pfeiffer RF: Magnetic resonance imaging studies in rapidly progressive Autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration. Neurodegeneration. 1992, 1: 217-224.
Murrell JR, Spillantini MG, Zolo P, Guazzelli M, Smith MJ, Hasegawa M, Redi F, Crowther RA, Pietrini P, Ghetti B, Goedert M: Tau gene mutation G389R causes a tauopathy with abundant pick body-like inclusions and axonal deposits. J Neuropathol Exp Neurol. 1999, 58: 1207-1226.
Wszolek ZK, Pfeiffer RF, Bhatt MH, Schelper RL, Cordes M, Snow BJ, Rodnitzky RL, Wolters EC, Arwert F, Calne DB: Rapidly progressive autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration. Ann Neurol. 1992, 32: 312-320. 10.1002/ana.410320303.
Yasuda M, Yokoyama K, Nakayasu T, Nishimura Y, Matsui M, Yokoyama T, Miyoshi K, Tanaka C: A Japanese patient with frontotemporal dementia and parkinsonism by a tau P301S mutation. Neurology. 2000, 55: 1224-1227.
Pal PK, Wszolek ZK, Kishore A, de la Fuente-Fernandez R, Sossi V, Uitti RJ, Dobko T, Stoessl AJ: Positron emission tomography in pallido-ponto-nigral degeneration (PPND) family (frontotemporal dementia with parkinsonism linked to chromosome 17 and point mutation in tau gene). Parkinsonism Relat Disord. 2001, 7: 81-88. 10.1016/S1353-8020(00)00026-2.
Wszolek ZK, Lagerlund TD, Steg RF, McManis PG: Clinical neurophysiologic findings in patients with rapidly progressive familial parkinsonism and dementia with pallido-ponto-nigral degeneration. Electroenceph Clin Neurophysiol. 1998, 107: 213-222. 10.1016/S0013-4694(98)00064-9.
Sperfeld AD, Collatz MB, Baier H, Palmbach M, Storch A, Schwarz J, Tatsch K, Reske S, Joosse M, Heutink P, Ludolph AC: FTDP-17: an early-onset phenotype with parkinsonism and epileptic seizures caused by a novel mutation. Ann Neurol. 1999, 46: 708-715. 10.1002/1531-8249(199911)46:5<708::AID-ANA5>3.0.CO;2-K.
Lynch T, Sano M, Marder KS, Bell KL, Foster NL, Defendini RF, Sima AA, Keohane C, Nygaard TG, Fahn S, Mayeux R, Rowland LP, Wilhelmsen KC: Clinical characteristics of a family with chromosome 17-linked disinhibition-dementia-parkinsonism-amyotrophy complex. Neurology. 1994, 44: 1878-1884.
Cheshire WP, Tsuboi Y, Wszolek ZK: Physiologic assessment of autonomic dysfunction in pallidopontonigral degeneration with N279K mutation in the tau gene on chromosome 17. Auton Neurosci. 2002, 102: 71-77. 10.1016/S1566-0702(02)00205-9.
Gemignani A, Pietrini P, Murrell JR, Glazier BS, Zolo P, Guazzelli M, Ghetti B: Slow wave and rem sleep mechanisms are differently altered in hereditary pick disease associated with the TAU G389R mutation. Arch Ital Biol. 2005, 143: 65-79.
McRae CA, Diem G, Yamazaki TG, Mitek A, Wszolek ZK: Interest in genetic testing in pallido-ponto-nigral degeneration (PPND): a family with frontotemporal dementia with Parkinsonism linked to chromosome 17. Eur J Neurol. 2001, 8: 179-183. 10.1046/j.1468-1331.2001.00198.x.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wszolek, Z.K., Tsuboi, Y., Ghetti, B. et al. Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Orphanet J Rare Dis 1, 30 (2006). https://doi.org/10.1186/1750-1172-1-30
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1750-1172-1-30