Abstract
Spinal cord injury (SCI) can lead to the destruction of the blood-spinal cord barrier (BSCB), causing various inflammatory cytokines, neutrophils, and macrophages to infiltrate the lesion area, resulting in secondary injury. Basement membranes (BMs) are maintained by all types of cells in the BSCB and contribute to BSCB maintenance. Perlecan is an important constituent of vascular BMs, maintaining vascular integrity and neuroprotection. However, it is not clear whether Perlecan is involved in BSCB repair after SCI. In this study, we found that Perlecan was specifically expressed in the BMs in the spinal cord and underwent degradation/remodeling after SCI. Subsequently, a CRISPR/Cas9-based SAM system was used to overexpress Perlecan in the injured spinal cord, resulting in significantly enhanced locomotor recovery and neural regeneration. Overexpression of Perlecan reduced BSCB permeability along with the neuroinflammatory response. Interestingly, Perlecan inhibited stress fiber formation by interacting with integrin β1 and inhibiting downstream ROCK/MLC signaling, resulting in reduced tight junctions (TJs) disassembly and improved BSCB integrity. Furthermore, the integrin receptor antagonist GRGDSP abolished the effects of Perlecan overexpression on BSCB permeability and TJs integrity. Overall, our findings suggest that Perlecan reduces BSCB permeability and the neuroinflammatory response by interacting with integrin β1 and inhibiting the downstream ROCK/MLC pathway to promote neurological recovery after SCI.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xue W et al (2020) Epidermal growth factor receptor-extracellular-regulated kinase blockade upregulates TRIM32 signaling cascade and promotes neurogenesis after spinal cord injury. Stem Cells 38(1):118–133
Kumar H et al (2017) Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol 54(5):3578–3590
Sofroniew MV (2018) Dissecting spinal cord regeneration. Nature 557(7705):343–350
Hu J et al (2016) Targeting the blood-spinal cord barrier: a therapeutic approach to spinal cord protection against ischemia-reperfusion injury. Life Sci 158:1–6
Nakamura K et al (2019) Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J Cell Biol 218(10):3506–3525
Thomsen MS, Routhe LJ, Moos T (2017) The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37(10):3300–3317
Takigawa T et al (2010) Separation of the perivascular basement membrane provides a conduit for inflammatory cells in a mouse spinal cord injury model. J Neurotrauma 27(4):739–751
Roberts J, Kahle MP, Bix GJ (2012) Perlecan and the blood-brain barrier: beneficial proteolysis? Front Pharmacol 3:155
Fukuda S et al (2004) Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35(4):998–1004
Kerever A et al (2014) Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res 12(2):492–505
Martinez JR, Dhawan A, Farach-Carson MC (2018) Modular proteoglycan Perlecan/HSPG2: mutations, phenotypes, and functions. Genes (Basel) 9(11):556
Xu L, Nirwane A, Yao Y (2019) Basement membrane and blood-brain barrier. Stroke Vasc Neurol 4(2):78–82
Lee B et al (2011) Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest 121(8):3005–3023
Kahle MP et al (2012) Perlecan domain V is upregulated in human brain arteriovenous malformation and could mediate the vascular endothelial growth factor effect in lesional tissue. NeuroReport 23(10):627–630
Trout AL et al (2021) Perlecan domain-V enhances neurogenic brain repair after stroke in mice. Transl Stroke Res 12(1):72–86
van Horssen J et al (2001) Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer’s disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains. Acta Neuropathol 102(6):604–614
Marques SA et al (2009) A simple, inexpensive and easily reproducible model of spinal cord injury in mice: morphological and functional assessment. J Neurosci Methods 177(1):183–193
Xie C et al (2020) Astrocytic YAP promotes the formation of glia scars and neural regeneration after spinal cord injury. J Neurosci 40(13):2644–2662
Joshi M, Fehlings MG (2002) Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma 19(2):175–90
Yang L et al (2021) Low expression of TRAF3IP2-AS1 promotes progression of NONO-TFE3 translocation renal cell carcinoma by stimulating N(6)-methyladenosine of PARP1 mRNA and downregulating PTEN. J Hematol Oncol 14(1):46
Konermann S et al (2015) Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517(7536):583–588
Wu J et al (2016) Recombinant osteopontin stabilizes smooth muscle cell phenotype via integrin receptor/integrin-linked kinase/Rac-1 pathway after subarachnoid hemorrhage in rats. Stroke 47(5):1319–1327
Suzuki H et al (2010) Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke 41(8):1783–1790
Gu Y et al (2019) Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav Immun 80:394–405
Demjen D et al (2004) Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med 10(4):389–395
Xu N et al (2015) A sensitive and reliable test instrument to assess swimming in rats with spinal cord injury. Behav Brain Res 291:172–183
Ma M et al (2001) Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp Neurol 169(2):239–254
Hill RL et al (2009) Anatomical and functional outcomes following a precise, graded, dorsal laceration spinal cord injury in C57BL/6 mice. J Neurotrauma 26(1):1–15
Ge X et al (2021) Exosomal miR-155 from M1-polarized macrophages promotes EndoMT and impairs mitochondrial function via activating NF-κB signaling pathway in vascular endothelial cells after traumatic spinal cord injury. Redox Biol 41:101932
Joshi HP et al (2020) CORM-2-solid lipid nanoparticles maintain integrity of blood-spinal cord barrier after spinal cord injury in rats. Mol Neurobiol 57(6):2671–2689
Ying X et al (2020) Water treadmill training attenuates blood-spinal cord barrier disruption in rats by promoting angiogenesis and inhibiting matrix metalloproteinase-2/9 expression following spinal cord injury. Fluids Barriers CNS 17(1):70
Xu J et al (2017) Ultrastructural features of neurovascular units in a rat model of chronic compressive spinal cord injury. Front Neuroanat 11:136
Freria CM et al (2017) Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J Neurosci 37(13):3568–3587
Yao Y (2019) Basement membrane and stroke. J Cereb Blood Flow Metab 39(1):3–19
Kaneko S et al (2006) A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med 12(12):1380–1389
Ren ZW et al (2019) Effect of exosomes derived from MiR-133b-modified ADSCs on the recovery of neurological function after SCI. Eur Rev Med Pharmacol Sci 23(1):52–60
Orr MB, Gensel JC (2018) Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics 15(3):541–553
Bartanusz V et al (2011) The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 70(2):194–206
Reinhold AK, Rittner HL (2017) Barrier function in the peripheral and central nervous system-a review. Pflugers Arch 469(1):123–134
Nag S et al (2019) Increased Expression of vascular endothelial growth factor-D following brain injury. Int J Mol Sci 20(7):1594
Nourhaghighi N et al (2003) Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Lab Invest 83(8):1211–1222
Prasain N, Stevens T (2009) The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77(1):53–63
Shi Y et al (2016) Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 7:10523
dos Remedios CG et al (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev 83(2):433–473
Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71(11):1018–1039
Engelhardt B (2011) β1-integrin/matrix interactions support blood-brain barrier integrity. J Cereb Blood Flow Metab 31(10):1969–1971
Izawa Y et al (2018) β1-integrin-matrix interactions modulate cerebral microvessel endothelial cell tight junction expression and permeability. J Cereb Blood Flow Metab 38(4):641–658
Deguchi Y et al (2002) Internalization of basic fibroblast growth factor at the mouse blood-brain barrier involves perlecan, a heparan sulfate proteoglycan. J Neurochem 83(2):381–389
Miller JD et al (1997) Localization of perlecan (or a perlecan-related macromolecule) to isolated microglia in vitro and to microglia/macrophages following infusion of beta-amyloid protein into rodent hippocampus. Glia 21(2):228–243
Grindel BJ et al (2014) Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol 36:64–76
Bix GJ, Gowing EK, Clarkson AN (2013) Perlecan domain V is neuroprotective and affords functional improvement in a photothrombotic stroke model in young and aged mice. Transl Stroke Res 4(5):515–523
Marcelo A, Bix G (2015) The potential role of perlecan domain V as novel therapy in vascular dementia. Metab Brain Dis 30(1):1–5
Daley WP, Peters SB, Larsen M (2008) Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121(Pt 3):255–264
Kyprianou C et al (2020) Basement membrane remodelling regulates mouse embryogenesis. Nature 582(7811):253–258
Gasche Y et al (2006) Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front Biosci 11:1289–1301
Rust R, Kaiser J (2017) Insights into the dual role of inflammation after spinal cord injury. J Neurosci 37(18):4658–4660
Liu WY et al (2015) Increasing the permeability of the blood-brain barrier in three different models in vivo. CNS Neurosci Ther 21(7):568–574
Dejana E, Tournier-Lasserve E, Weinstein BM (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell 16(2):209–221
Nieuwenhuis B et al (2018) Integrins promote axonal regeneration after injury of the nervous system. Biol Rev Camb Philos Soc 93(3):1339–1362
Tang J et al (2020) TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin β 1 complex and regulating downstream FAK/RhoA signaling. Acta Pharm Sin B 10(6):987–1003
Acknowledgements
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This work is supported by the Natural Science Foundation of Guangdong Province (2017A030312009, 2017A030313111), the National Natural Science Foundation (82071386, 81974329, 81672140), Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104008), and Research Grant of Guangdong Province Key Laboratory of Psychiatric Disorders (N201904).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Lixin Zhu and Jiasong Guo designed the study. Material preparation, data collection and analysis were performed by Changnan Xie, Yihan Wang, Jinfeng Wang, Yizhou Xu and Haining Liu. The first draft of the manuscript was written by Changnan Xie and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All animal experiments were performed in accordance with Institutional Animal Care and Use committee guidelines and approved protocols at the Southern Medical University (ethics Number: LAEC-2022–064).
Consent to Participate
Not applicable.
Consent for Publication
All data generated or analyzed during this study are included in this published article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplemental Fig. 1
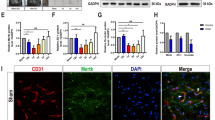
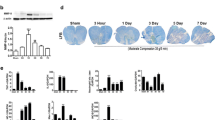
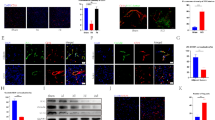
The expression effect of Perlecan by SAM system in injured spinal cords A and B Real time PCR analysis showed the relative mRNA level of Perlecan in injured spinal cords from SCI, LV-GFP and LV-Perlecan groups at 7 and 14 days after SCI (n = 3 animal per group). C Immunostaining of Perlecan in spinal cords from SCI, LV-GFP and LV-Perlecan groups at 14 days after SCI. White dashed lines indicated the injury sites. LC indicated lesion core. Quantitative data in (A and B) were analyzed using one-way ANOVA with Dunnett’s multiple comparison test. **p<0.01, compared with LV-Perlecan group. Data were mean ± SEM. Scale bars, 20 μm (PNG 3.26 MB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, C., Wang, Y., Wang, J. et al. Perlecan Improves Blood Spinal Cord Barrier Repair Through the Integrin β1/ROCK/MLC Pathway After Spinal Cord Injury. Mol Neurobiol 60, 51–67 (2023). https://doi.org/10.1007/s12035-022-03041-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03041-9