Abstract
Purpose of Review
Schizophrenia (SZ) is a debilitating mental illness; existing treatments are partially effective and associated with significant side effect burden, largely due to our limited understanding of disease mechanisms and the trajectory of disease progression. Accumulating evidence suggests that metabolic changes associated with glucose metabolism, mitochondrial dysfunction, and redox imbalance play an important role in the pathophysiology of schizophrenia. However, the molecular mechanisms associated with these abnormalities in the brains of schizophrenia patients and the ways in which they change over time remain unclear. This paper aims to review the current literature on molecular mechanisms and in vivo magnetic resonance spectroscopy (MRS) studies of impaired energy metabolism in patients at clinical high risk for psychosis, with first-episode SZ, and with chronic SZ. Our review covers research related to high-energy phosphate metabolism, lactate, intracellular pH, redox ratio, and the antioxidant glutathione.
Recent Findings
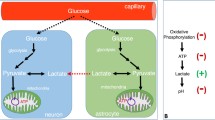
Both first-episode and chronic SZ patients display a significant reduction in creatine kinase reaction activity and redox (NAD + /NADH) ratio in the prefrontal cortex. Chronic, but not first-episode, SZ patients also show a trend toward increased lactate levels and decreased pH value. These findings suggest a progressive shift from oxidative phosphorylation to glycolysis for energy production over the course of SZ, which is associated with redox imbalance and mitochondrial dysfunction.
Summary
Accumulating evidence indicates that aberrant brain energy metabolism associated with mitochondrial dysfunction and redox imbalance plays a critical role in SZ and will be a promising target for future treatments.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
McCutcheon RA, Keefe RSE, McGuire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry. 2023.
Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519–34.
Goff DC, Li C, Thorpe L. Does early intervention improve the long-term course of schizophrenia? Am J Psychiatry. 2020;177(4):288–90.
Lally J, et al. Remission and recovery from first-episode psychosis in adults: systematic review and meta-analysis of long-term outcome studies. Br J Psychiatry. 2017;211(6):350–8.
AlAqeel B, Margolese HC. Remission in schizophrenia: critical and systematic review. Harv Rev Psychiatry. 2013;20(6):281–97.
Cuenod M, et al. Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol Psychiatry. 2022;27(4):1886–97.
Steullet P, et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? Schizophr Res. 2016;176(1):41–51.
Demjaha A, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017;47(11):1981–9.
Kaar SJ, et al. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704.
McCutcheon RA, Marques TR, Howes OD. Schizophrenia—an overview. JAMA Psychiat. 2020;77(2):201–10.
• Perkins DO, Jeffries CD, Do KQ. Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. 2020. This paper provides a summary of the evidence supporting redox dysregulation as a pathological mechanism driving the development of psychosis.
Hardingham GE, Do KQ. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci. 2016;17(2):125–34.
Henkel ND, et al. Schizophrenia: a disorder of broken brain bioenergetics. Mol Psychiatry. 2022;27(5):2393–404.
Balasubramanian V. Brain power. Proc Natl Acad Sci. 2021;118(32):e2107022118.
Roberts RC. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion. 2021;56:91–101.
Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426.
Hyder F, et al. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab. 2013;33(3):339–47.
Kowalczyk P, et al. Mitochondrial oxidative stress-a causative factor and therapeutic target in many diseases. Int J Mol Sci. 2021;22(24).
Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiat. 2013;74(6):400–9.
Hjelm BE, et al. Evidence of mitochondrial dysfunction within the complex genetic etiology of schizophrenia. Complex Psychiatry. 2015;1(4):201–19.
Zuccoli GS, et al. Mitochondrial, cell cycle control and neuritogenesis alterations in an iPSC-based neurodevelopmental model for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023:1–16.
da Silveira Paulsen B, et al. Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant. 2012;21(7):1547–59.
Kathuria A, et al. Disease-specific differences in gene expression, mitochondrial function and mitochondria-endoplasmic reticulum interactions in iPSC-derived cerebral organoids and cortical neurons in schizophrenia and bipolar disorder. Discover Mental Health. 2023;3(1):8.
Townsend L, et al. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of 18FDG-PET studies in schizophrenia. Psychol Med. 2022:1–18.
Chouinard VA, et al. Impaired insulin signaling in unaffected siblings and patients with first-episode psychosis. Mol Psychiatry. 2019;24(10):1513–22.
Yuksel C, et al. Phosphorus magnetic resonance spectroscopy studies in schizophrenia. J Psychiatr Res. 2015;68:157–66.
• Du F, et al. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiat. 2014;71(1):19–27. This paper uses a novel 31P-MT-MRS approach to examine creatine kinase reaction rate and intracellular pH in vivo in chronic schizophrenia pateints. Creatine kinase rate and intracellular pH were significnatly reduced in schizophrenia pateints compared to controls, indicating bionenergetic abnormalities.
Dean B, et al. Evidence for impaired glucose metabolism in the striatum, obtained postmortem, from some subjects with schizophrenia. Transl Psychiatry. 2016;6(11):e949–e949.
Prabakaran S, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9(7):684–97.
Pruett BS, Meador-Woodruff JH. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: a focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr Res. 2020;223:29–42.
Rowland LM, et al. Elevated brain lactate in schizophrenia: a 7 T magnetic resonance spectroscopy study. Transl Psychiatry. 2016;6(11):e967.
Wijtenburg SA, et al. Metabolite alterations in adults with schizophrenia, first degree relatives, and healthy controls: a multi-region 7T MRS study. Front Psychiatry. 2021;12:656459.
Wang AM, et al. Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiat. 2019;76(3):314–23.
Wang M, et al. Longitudinal changes in brain metabolites in healthy controls and patients with first episode psychosis: a 7-Tesla MRS study. Mol Psychiatry. 2023.
Da Silva T, et al. Glutathione, the major redox regulator, in the prefrontal cortex of individuals at clinical high risk for psychosis. Int J Neuropsychopharmacol. 2018;21(4):311–8.
Park H-J, Choi I, Leem K-H. Decreased brain pH and pathophysiology in schizophrenia. Int J Mol Sci. 2021;22(16):8358.
Dogan AE, et al. Brain lactate and pH in schizophrenia and bipolar disorder: a systematic review of findings from magnetic resonance studies. Neuropsychopharmacology. 2018;43(8):1681–90.
Romeo B, et al. Magnetic resonance spectroscopy studies in subjects with high risk for psychosis: A meta-analysis and review. J Psychiatr Res. 2020;125:52–65.
Du F, et al. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–14.
Du F, et al. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105(17):6409–14.
Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab. 2014;34(8):1270–82.
Kole K, et al. Parvalbumin basket cell myelination accumulates axonal mitochondria to internodes. Nat Commun. 2022;13(1):7598.
Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11(4):275–83.
Chouinard V-A, et al. Brain bioenergetics and redox state measured by 31P magnetic resonance spectroscopy in unaffected siblings of patients with psychotic disorders. Schizophr Res. 2017;187:11–6.
• Yuksel C, et al. Abnormal brain bioenergetics in first-episode psychosis. Schizophr Bull Open. 2021;2(1). This study used 31P-MT-MRS to reveal a decrease in creatine kinase reaction rates in first-episode schizophrenia, building upon previous research that identified the same abnormality in chronic schizophrenia patients.
Du F, et al. Abnormalities in high-energy phosphate metabolism in first-episode bipolar disorder measured using 31P-magnetic resonance spectroscopy. Biol Psychiat. 2018;84(11):797–802.
Kim SY, et al. Rapid and simultaneous measurement of phosphorus metabolite pool size ratio and reaction kinetics of enzymes in vivo. J Magn Reson Imaging. 2018;47(1):210–21.
Chen W, et al. Increase of creatine kinase activity in the visual cortex of human brain during visual stimulation: a 31P NMR magnetization transfer study. Magn Reson Med. 1997;38(4):551–7.
Kašparová S, et al. A study of creatine kinase reaction in rat brain under chronic pathological conditions—chronic ischemia and ethanol intoxication. Brain Res Bull. 2000;53(4):431–5.
Mlynárik V, et al. Creatine kinase reaction rates in rat brain during chronic ischemia. Magn Reson Mater Phys, Biol Med. 1998;7:162–5.
Du F, et al. In vivo proton MRS to quantify anesthetic effects of pentobarbital on cerebral metabolism and brain activity in rat. Magn Reson Med. 2009;62(6):1385–93.
Chaumeil MM, et al. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A. 2009;106(10):3988–93.
Song X, et al. Bioenergetics and abnormal functional connectivity in psychotic disorders. Mol Psychiatry. 2021;26(6):2483–92.
Zhao RZ, et al. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med. 2019;44(1):3–15.
Dwir D, et al. Redox and immune signaling in schizophrenia: new therapeutic potential. Int J Neuropsychopharmacol. 2023;26(5):309–21.
Khadimallah I, et al. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2022;27(2):1192–204.
Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–71.
Das TK, et al. Antioxidant defense in schizophrenia and bipolar disorder: a meta-analysis of MRS studies of anterior cingulate glutathione. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:94–102.
Sydnor VJ, Roalf DR. A meta-analysis of ultra-high field glutamate, glutamine, GABA and glutathione 1HMRS in psychosis: implications for studies of psychosis risk. Schizophr Res. 2020;226:61–9.
MacKinley M, et al. Central oxidative stress and early vocational outcomes in first episode psychosis: a 7-Tesla Magnetic Resonance Spectroscopy study of glutathione. Schizophr Bull. 2022;48(4):921–30.
Coughlin JM, et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol Psychiatry. 2021;26(7):3502–11.
Ravanfar P, et al. In vivo 7-Tesla MRI investigation of brain iron and its metabolic correlates in chronic schizophrenia. Schizophrenia. 2022;8(1):86.
Iwata Y, et al. Glutathione levels and glutathione-glutamate correlation in patients with treatment-resistant schizophrenia. Schizophr Bull Open. 2021;2(1):sgab006.
Jeon P, et al. Glutathione as a molecular marker of functional impairment in patients with at-risk mental state: 7-Tesla 1H-MRS Study. Brain Sci. 2021;11(7):941.
Da Silva T, et al. Mitochondrial function in individuals at clinical high risk for psychosis. Sci Rep. 2018;8(1):6216.
Bonora M, et al. ATP synthesis and storage. Purinergic Signal. 2012;8(3):343–57.
• Kim S-Y, et al. Redox dysregulation in schizophrenia revealed by in vivo NAD+/NADH measurement. Schizophr Bull. 2017;43(1):197–204. This study uses MRS to reveal significant redox dysregulation (reduced NAD+/NADH) in both first-episode and chronic schizophrenia pateints.
Xiao W, Loscalzo J. Metabolic responses to reductive stress. Antioxid Redox Signal. 2020;32(18):1330–47.
Skupienski R, Do KQ, Xin L. In vivo (31)P magnetic resonance spectroscopy study of mouse cerebral NAD content and redox state during neurodevelopment. Sci Rep. 2020;10(1):15623.
Skupienski R, et al. Developmental changes in cerebral NAD and neuroenergetics of an antioxidant compromised mouse model of schizophrenia. bioRxiv. 2022.
Lushchak VI, Storey KB. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. Excli J. 2021;20:956–67.
Clay HB, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311–24.
Aoyama K. Glutathione in the brain. Int J Mol Sci. 2021;22(9).
Sies H, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23(7):499–515.
Shadel GS, Horvath TL. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–9.
Kim SY, et al. In vivo brain glycine and glutamate concentrations in patients with first-episode psychosis measured by echo time-averaged proton magnetic resonance spectroscopy at 4T. Biol Psychiatry. 2018;83(6):484–91.
Mladenov M, et al. Oxidative stress, reductive stress and antioxidants in vascular pathogenesis and aging. Antioxidants (Basel). 2023;12(5).
Merritt K, et al. Association of age, antipsychotic medication, and symptom severity in schizophrenia with proton magnetic esonance spectroscopy brain glutamate level: a mega-analysis of individual participant-level data. JAMA Psychiat. 2021;78(6):667–81.
Bustillo JR, et al. (1)H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2009.
Theberge J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159(11):1944–6.
Merritt K, et al. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiat. 2016;73(7):665–74.
Egerton A, et al. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal (1)H-MRS studies. Front Psychiatry. 2017;8:66.
Brandt AS, et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a (1)H MRS study at 7 Tesla. Schizophr Res. 2016;172(1–3):101–5.
Smucny J, Carter CS, Maddock RJ. Medial prefrontal cortex glutamate is reduced in schizophrenia and moderated by measurement quality: a meta-analysis of proton magnetic resonance spectroscopy studies. Biol Psychiatry. 2021;90(9):643–51.
Napoli E, et al. Mitochondrial citrate transporter-dependent metabolic signature in the 22q11.2 deletion syndrome. J Biol Chem. 2015;290(38):23240–53.
Cleynen I, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. 2021;26(8):4496–510.
Covarrubias AJ, et al. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41.
Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull. 2017;44(2):398–408.
Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5(4):256–62.
Smaga I, Frankowska M, Filip M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br J Pharmacol. 2021;178(13):2569–94.
Conus P, et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. Schizophr Bull. 2018;44(2):317–27.
Reiten OK, et al. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199:111567.
Acknowledgements
The authors thank our volunteers and Mr. Elliot Kuan and Ms. Margaret Gardner for their assistance in the experiments and subject recruitment; Drs. Xiaopeng Song, Xi Chen, Cagri Yuksel, Virginie-Anne Chouinard, Kim Do Cuenod, and Bruce Cohen for their thoughtful discussions.
Funding
This research work was supported by National Institutes of Health (NIH) grants: R21MH114020, R01MH114982, P50MH115846, K24MH104449, R01AG066670, and R01MH095809.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Over the past 3 years, Dr. Dost Ongur has received honoraria from Neumora Inc. and Guggenheim LLC for scientific presentations. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. None of the other authors have conflict of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stein, A., Zhu, C., Du, F. et al. Magnetic Resonance Spectroscopy Studies of Brain Energy Metabolism in Schizophrenia: Progression from Prodrome to Chronic Psychosis. Curr Psychiatry Rep 25, 659–669 (2023). https://doi.org/10.1007/s11920-023-01457-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11920-023-01457-1