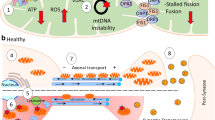
Abstract
Alzheimer’s disease (AD) and type 2 diabetes (T2D) are known to be correlated in terms of their epidemiology, histopathology, and molecular and biochemical characteristics. The prevalence of T2D leading to AD is approximately 50–70%. Moreover, AD is often considered type III diabetes because of the common risk factors. Uncontrolled T2D may affect the brain, leading to memory and learning deficits in patients. In addition, metabolic disorders and impaired oxidative phosphorylation in AD and T2D patients suggest that mitochondrial dysfunction is involved in both diseases. The dysregulation of pathways involved in maintaining mitochondrial dynamics, biogenesis and mitophagy are responsible for exacerbating the impact of hyperglycemia on the brain and neurodegeneration under T2D conditions. The first section of this review describes the recent views on mitochondrial dysfunction that connect these two disease conditions, as the pathways are observed to overlap. The second section of the review highlights the importance of different mitochondrial miRNAs (mitomiRs) involved in the regulation of mitochondrial dynamics and their association with the pathogenesis of T2D and AD. Therefore, targeting mitochondrial biogenesis and mitophagy pathways, along with the use of mitomiRs, could be a potent therapeutic strategy for T2D-related AD. The last section of the review highlights the known drugs targeting mitochondrial function for the treatment of both disease conditions.


Similar content being viewed by others
References
Li X, Leng S, Song D (2015) Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clinical Interventions in Aging 549
Jayaraman A, Pike CJ (2014) Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Current Diabetes Reports 14:476
Lu F-P, Lin K-P, Kuo H-K (2009) Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One 4:e4144
Xia W, Wang S, Sun Z, Bai F, Zhou Y, Yang Y, Wang P, Huang Y et al (2013) Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology 38:2493–2501
Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Munch G, Wood AG et al (2013) Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36:4036–4042
Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, del Prato S, Rabuazzo AM et al (2005) Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 48:282–289
Albensi BC (2019) Dysfunction of mitochondria: implications for Alzheimer’s disease. Int Rev Neurobiol 145:13–27
Hauptmann S, Scherping I, Dröse S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A et al (2009) Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 30:1574–1586
Reddy AP, Reddy PH (2017) Mitochondria-targeted molecules as potential drugs to treat patients with Alzheimer’s disease. Prog Mol Biol Transl Sci 146:173–201
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Vos SJB, Verhey F, Frölich L, Kornhuber J, Wiltfang J, Maier W, Peters O, Rüther E et al (2015) Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain 138:1327–1338
Nooyens ACJ, Baan CA, Spijkerman AMW, Verschuren WMM (2010) Type 2 diabetes and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Diabetes Care 33:1964–1969
Archer SL (2013) Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369:2236–2251
Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39:503–536
Flippo KH, Strack S (2017) Mitochondrial dynamics in neuronal injury, development and plasticity. J Cell Sci 130:671–681
Tilokani L, Nagashima S, Paupe V, Prudent J (2018) Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 62:341–360
Williams M, Caino MC (2018) Mitochondrial dynamics in type 2 diabetes and cancer. Front Endocrinol 9:211
Eisner V, Picard M, Hajnóczky G (2018) Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol 20:755–765
Ding W-X, Yin X-M (2012) Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem 393:547–564
Burgos-Morón E, Abad-Jiménez Z, Marañón AM de, et al (2019) Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med Res 8 https://doi.org/10.3390/jcm8091385
Mariani E, Polidori MC, Cherubini A, Mecocci P (2005) Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Anal Technol Biomed Life Sci 827:65–75
Winiarska-Mieczan A, Baranowska-Wójcik E, Kwiecień M, Grela ER, Szwajgier D, Kwiatkowska K, Kiczorowska B (2020) The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 12. https://doi.org/10.3390/nu12020435
Cadonic C, Sabbir MG, Albensi BC (2016) Mechanisms of mitochondrial dysfunction in Alzheimer’s disease. Mol Neurobiol 53:6078–6090
Perez Ortiz JM, Swerdlow RH (2019) Mitochondrial dysfunction in Alzheimer’s disease: role in pathogenesis and novel therapeutic opportunities. Br J Pharmacol 176:3489–3507
Cash AD, Perry G, Ogawa O, Raina AK, Zhu X, Smith MA (2002) Book review: is Alzheimer’s disease a mitochondrial disorder? Neuroscientist 8:489–496
Bhatti JS, Bhatti GK, Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol basis Dis 1863:1066–1077
Olokoba AB, Obateru OA, Olokoba LB (2012) Type 2 diabetes mellitus: a review of current trends. Oman medical journal 27:269–273
DeFronzo RA, Ferrannini E, Groop L et al (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1:15019
Priyadarshini M, Kamal MA, Greig NH et al (2012) Alzheimer’s disease and type 2 diabetes: exploring the association to obesity and tyrosine hydroxylase. CNS Neurol Disord Drug Targets 11:482–489
Barbagallo M (2014) Type 2 diabetes mellitus and Alzheimer’s disease. World J Diabetes 5:889–893
Onyango IG, Dennis J, Khan SM (2016) Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis 7:201–214
Santiago JA, Bottero V, Potashkin JA (2019) Transcriptomic and network analysis highlight the association of diabetes at different stages of Alzheimer’s disease. Front Neurosci 13
Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9:119–126
Sajjad R, Arif R, Shah AA, et al (2018) Pathogenesis of Alzheimer’s disease: role of amyloid-beta and hyperphosphorylated Tau protein. Indian Journal of Pharmaceutical Sciences 80
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403
Hamley IW (2012) The amyloid beta peptide: a chemist’s perspective. Role in Alzheimer’s and fibrillization Chemical Reviews 112:5147–5192
Chen G-F, Xu T-H, Yan Y, Zhou YR, Jiang Y, Melcher K, Xu HE (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin 38:1205–1235
Devi L (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci 26:9057–9068
Walsh DM, Selkoe DJ (2004) Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron 44:181–193
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440:352–357
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57:1105–1121
Hao K, Di Narzo AF, Ho L et al (2015) Shared genetic etiology underlying Alzheimer’s disease and type 2 diabetes. Mol Asp Med 43-44:66–76
Vieira MNN, Lima-Filho RAS, De Felice FG (2018) Connecting Alzheimer’s disease to diabetes: underlying mechanisms and potential therapeutic targets. Neuropharmacology 136:160–171
Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R (2007) Relation of diabetes to mild cognitive impairment. Arch Neurol 64:570–575
Gao Y, Xiao Y, Miao R, Zhao J, Cui M, Huang G, Fei M (2016) The prevalence of mild cognitive impairment with type 2 diabetes mellitus among elderly people in China: a cross-sectional study. Arch Gerontol Geriatr 62:138–142
Li W, Wang T, Xiao S (2016) Type 2 diabetes mellitus might be a risk factor for mild cognitive impairment progressing to Alzheimer’s disease. Neuropsychiatr Dis Treat 12:2489–2495
Brands AMA, Biessels GJ, de Haan EHF, Kappelle LJ, Kessels RPC (2005) The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 28:726–735
Duarte JMN, Agostinho PM, Carvalho RA, Cunha RA (2012) Caffeine consumption prevents diabetes-induced memory impairment and synaptotoxicity in the hippocampus of NONcZNO10/LTJ mice. PLoS One 7:e21899
Li W, Sun L, Li G, Xiao S (2019) Prevalence, influence factors and cognitive characteristics of mild cognitive impairment in type 2 diabetes mellitus. Front Aging Neurosci 11:180
Liu J, Liu T, Wang W, Ma L, Ma X, Shi S, Gong Q, Wang M (2017) Reduced gray matter volume in patients with type 2 diabetes mellitus. Front Aging Neurosci 9:161
Angajala A, Lim S, Phillips JB, Kim JH, Yates C, You Z, Tan M (2018) Diverse roles of mitochondria in immune responses: novel insights into immuno-metabolism. Front Immunol 9:1605
Reis F, Soares, Nunes, Pereira (2012) Diabetic encephalopathy: the role of oxidative stress and inflammation in type 2 diabetes. International Journal of Interferon, Cytokine and Mediator Research 75
Ortiz-Avila O, Esquivel-Martínez M, Olmos-Orizaba BE et al (2015) Avocado oil improves mitochondrial function and decreases oxidative stress in brain of diabetic rats. J Diabetes Res 2015:485759
Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI (2012) Metabolic alterations induced by sucrose intake and Alzheimer’s disease promote similar brain mitochondrial abnormalities. Diabetes 61:1234–1242
Carvalho C, Machado N, Mota PC, Correia SC, Cardoso S, Santos RX, Santos MS, Oliveira CR et al (2013) Type 2 diabetic and Alzheimer’s disease mice present similar behavioral, cognitive, and vascular anomalies. J Alzheimers Dis 35:623–635
Westermann B (2010) Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884
Losón OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24:659–667
Rovira-Llopis S, Bañuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM (2017) Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 11:637–645
Ježek P, Dlasková A (2019) Dynamic of mitochondrial network, cristae, and mitochondrial nucleoids in pancreatic β-cells. Mitochondrion 49:245–258
Moreira PI (2012) Alzheimer’s disease and diabetes: an integrative view of the role of mitochondria, oxidative stress, and insulin. J Alzheimers Dis 30(Suppl 2):S199–S215
Kiritoshi S, Nishikawa T, Sonoda K, Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H et al (2003) Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes 52:2570–2577
Ashrafi G, Ryan TA (2017) Glucose metabolism in nerve terminals. Curr Opin Neurobiol 45:156–161
Chow H-M, Shi M, Cheng A, Gao Y, Chen G, Song X, So RWL, Zhang J et al (2019) Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci 22:1806–1819
Lenzen S (2017) Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim Biophys Acta Gen Subj 1861:1929–1942
Quirós PM, Ramsay AJ, Sala D, Fernández-Vizarra E, Rodríguez F, Peinado JR, Fernández-García MS, Vega JA et al (2012) Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31:2117–2133
Sebastián D, Hernández-Alvarez MI, Segalés J et al (2012) Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A 109:5523–5528
Zorzano A, Liesa M, Palacín M (2009) Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch Physiol Biochem 115:1–12
Yu T, Sheu S-S, Robotham JL, Yoon Y (2008) Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79:341–351
Perry G, Zhu X, Smith MA (2013) Alzheimer’s disease: advances for a new century. IOS Press
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Teleanu RI, Chircov C, Grumezescu AM, Volceanov A, Teleanu DM (2019) Antioxidant therapies for neuroprotection—a review. J Clin Med Res 8:8. https://doi.org/10.3390/jcm8101659
Shoffner JM (1997) Oxidative phosphorylation defects and Alzheimer’s disease. Neurogenetics 1:13–19
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. NeuroMolecular Med 5:147–162
Chornenkyy Y, Wang W, Wei A, Nelson PT (2019) Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol 29:3–17
Oliver D, Reddy P (2019) Dynamics of dynamin-related protein 1 in Alzheimer’s disease and other neurodegenerative diseases. Cells 8:961
Wang X, Su B, Lee H-G, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29:9090–9103
Calkins MJ, Reddy PH (2011) Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochim Biophys Acta 1812:507–513
Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20:2495–2509
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B et al (2010) Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20:S609–S631
Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173:470–482
Santos RX, Correia SC, Alves MG, Oliveira PF, Cardoso S, Carvalho C, Seiça R, Santos MS et al (2014) Mitochondrial quality control systems sustain brain mitochondrial bioenergetics in early stages of type 2 diabetes. Mol Cell Biochem 394:13–22
Leinninger GM, Backus C, Sastry AM, Yi YB, Wang CW, Feldman EL (2006) Mitochondria in DRG neurons undergo hyperglycemic mediated injury through Bim, Bax and the fission protein Drp1. Neurobiol Dis 23:11–22
Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, Feldman EL (2010) Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 53:160–169
Vincent AM, Edwards JL, McLean LL et al (2010) Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol 120:477–489
Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, Hu G, Sosunov AA et al (2015) Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes 64:1728–1742
Liu J, Shen W, Zhao B, Wang Y, Wertz K, Weber P, Zhang P (2009) Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv Drug Deliv Rev 61:1343–1352
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P et al (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458:1056–1060
Anderson RM, Barger JL, Edwards MG, Braun KH, O’Connor CE, Prolla TA, Weindruch R (2008) Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 7:101–111
Hoeks J, Hesselink MKC, Russell AP, Mensink M, Saris WHM, Mensink RP, Schrauwen P (2006) Peroxisome proliferator-activated receptor-γ coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia 49:2419–2426
Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS (2002) Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296:349–352
Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC et al (2014) Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28:2705–2714
Zhao Z, Pu Y (2019) Lixisenatide enhances mitochondrial biogenesis and function through regulating the CREB/PGC-1α pathway. Biochem Biophys Res Commun 508:1120–1125
Carvalho C, Santos MS, Oliveira CR, Moreira PI (2015) Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta (BBA) - Mol Basis Dis 1852:1665–1675
Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X (2012) Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem 120:419–429
Wang C-F, Song C-Y, Wang X et al (2019) Protective effects of melatonin on mitochondrial biogenesis and mitochondrial structure and function in the HEK293-APPswe cell model of Alzheimer’s disease. Eur Rev Med Pharmacol Sci 23:3542–3550
Chakravorty A, Jetto CT, Manjithaya R (2019) Dysfunctional mitochondria and mitophagy as drivers of Alzheimer’s disease pathogenesis. Frontiers in Aging Neuroscience 11
Silzer T, Barber R, Sun J, Pathak G, Johnson L, O’Bryant S, Phillips N (2019) Circulating mitochondrial DNA: new indices of type 2 diabetes-related cognitive impairment in Mexican Americans. PLoS One 14:e0213527
Agrawal R, Zhuang Y, Cummings BP, Stanhope KL, Graham JL, Havel PJ, Gomez-Pinilla F (2014) Deterioration of plasticity and metabolic homeostasis in the brain of the UCD-T2DM rat model of naturally occurring type-2 diabetes. Biochim Biophys Acta 1842:1313–1323
Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB (2007) Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmacol 225:214–220
Raza H, John A, Howarth FC (2015) Increased oxidative stress and mitochondrial dysfunction in zucker diabetic rat liver and brain. Cell Physiol Biochem 35:1241–1251
Monette MCE, Baird A, Jackson DL (2014) A meta-analysis of cognitive functioning in nondemented adults with type 2 diabetes mellitus. Can J Diabetes 38:401–408
Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, Sweatt JD (2012) Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem 98:25–32
Peeri M, Amiri S (2015) Protective effects of exercise in metabolic disorders are mediated by inhibition of mitochondrial-derived sterile inflammation. Med Hypotheses 85:707–709
Twig G, Shirihai OS (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14:1939–1951
Liu L, Sakakibara K, Chen Q, Okamoto K (2014) Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res 24:787–795
Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446
Smirnova E, Griparic L, Shurland D-L, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12:2245–2256
Zhu J, Wang KZQ, Chu CT (2013) After the banquet. Autophagy 9:1663–1676
Beck JS, Mufson EJ, Counts SE (2016) Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res 13:610–614
Park S, Choi S-G, Yoo S-M, Son JH, Jung YK (2014) Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy 10:1906–1920
Wu H, Wang Y, Li W, Chen H, du L, Liu D, Wang X, Xu T et al (2019) Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 15:1882–1898
Sato S, Furuya N (2018) Induction of PINK1/Parkin-mediated mitophagy. Methods Mol Biol 1759:9–17
Chen G, Kroemer G, Kepp O (2020) Mitophagy: an emerging role in aging and age-associated diseases. Front Cell Dev Biol 8:200
Ordureau A, Heo J-M, Duda DM, Paulo JA, Olszewski JL, Yanishevski D, Rinehart J, Schulman BA et al (2015) Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci U S A 112:6637–6642
McLelland G-L, Goiran T, Yi W, Dorval G, Chen CX, Lauinger ND, Krahn AI, Valimehr S et al (2018) Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. Elife 7. https://doi.org/10.7554/eLife.32866
Bhansali S, Bhansali A, Walia R, Saikia UN, Dhawan V (2017) Alterations in mitochondrial oxidative stress and mitophagy in subjects with prediabetes and type 2 diabetes mellitus. Front Endocrinol 8:347
Gustafsson ÅB, Dorn GW (2019) Evolving and expanding the roles of mitophagy as a homeostatic and pathogenic process. Physiol Rev 99:853–892
Hernández MG, Aguilar AG, Burillo J, Oca RG, Manca MA, Novials A, Alcarraz-Vizan G, Guillén C et al (2018) Pancreatic β cells overexpressing hIAPP impaired mitophagy and unbalanced mitochondrial dynamics. Cell Death Dis 9:481
Yang S, Xia C, Li S, du L, Zhang L, Zhou R (2014) Defective mitophagy driven by dysregulation of rheb and KIF5B contributes to mitochondrial reactive oxygen species (ROS)-induced nod-like receptor 3 (NLRP3) dependent proinflammatory response and aggravates lipotoxicity. Redox Biol 3:63–71
Seillier M, Pouyet L, N’Guessan P et al (2015) Defects in mitophagy promote redox-driven metabolic syndrome in the absence of TP 53 INP 1. EMBO Molecular Medicine 7:802–818
Gundersen AE, Kugler BA, McDonald PM et al (2020) Altered mitochondrial network morphology and regulatory proteins in mitochondrial quality control in myotubes from severely obese humans with or without type 2 diabetes. Appl Physiol Nutr Metab 45:283–293
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM et al (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22:401–412
Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40:151–166
Reddy PH, Oliver DM (2019) Amyloid beta and phosphorylated Tau-induced defective autophagy and mitophagy in Alzheimer’s disease. Cells 8:8. https://doi.org/10.3390/cells8050488
Castellazzi M, Patergnani S, Donadio M, et al (2019) Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Scientific Reports 9
Caberlotto L, Phuong Nguyen T, Lauria M et al (2019) Cross-disease analysis of Alzheimer’s disease and type-2 diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep 9:3965
Khang R, Park C, Shin J-H (2015) Dysregulation of parkin in the substantia nigra of db/db and high-fat diet mice. Neuroscience 294:182–192
Jin H, Zhu Y, Li Y, Ding X, Ma W, Han X, Wang B (2019) BDNF-mediated mitophagy alleviates high-glucose-induced brain microvascular endothelial cell injury. Apoptosis 24:511–528
Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L (2014) Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Frontiers in Cellular Neuroscience 8
Reddy PH, Tonk S, Kumar S, Vijayan M, Kandimalla R, Kuruva CS, Reddy AP (2017) A critical evaluation of neuroprotective and neurodegenerative MicroRNAs in Alzheimer’s disease. Biochem Biophys Res Commun 483:1156–1165
Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S et al (2011) Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One 6:e17850
Nicolas FE, Lopez-Martinez AF (2010) MicroRNAs in human diseases. Recent Patents on DNA & Gene Sequences 4:142–154
Peters L, Meister G (2007) Argonaute proteins: mediators of RNA silencing. Mol Cell 26:611–623
Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E (2008) Deadenylation is a widespread effect of miRNA regulation. RNA 15:21–32
John A, Kubosumi A, Reddy PH (2020) Mitochondrial MicroRNAs in aging and neurodegenerative diseases. Cells 9 https://doi.org/10.3390/cells9061345
Li X, Wang Z, Tan L, Wang Y, Lu C, Chen R, Zhang S, Gao Y et al (2017) Correcting miR92a-vGAT-mediated GABAergic dysfunctions rescues human Tau-induced anxiety in mice. Mol Ther 25:140–152
Weinberg RB, Mufson EJ, Counts SE (2015) Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front Neurosci 9:430
Dehwah MAS, Xu A, Huang Q (2012) MicroRNAs and type 2 diabetes/obesity. Journal of Genetics and Genomics 39:11–18
Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM (2008) Conditional loss of dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci 28:4322–4330
Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, McManus MT (2008) Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc Natl Acad Sci 105:5614–5619
Ma X, Liu L, Meng J (2017) MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett 661:57–62
Thounaojam MC, Jadeja RN, Warren M, Powell FL, Raju R, Gutsaeva D, Khurana S, Martin PM et al (2019) MicroRNA-34a (miR-34a) Mediates retinal endothelial cell premature senescence through mitochondrial dysfunction and loss of antioxidant activities. Antioxidants (Basel) 8. https://doi.org/10.3390/antiox8090328
Docrat TF, Nagiah S, Naicker N, Baijnath S, Singh S, Chuturgoon AA (2020) The protective effect of metformin on mitochondrial dysfunction and endoplasmic reticulum stress in diabetic mice brain. Eur J Pharmacol 875:173059
Xie Y, Chu A, Feng Y, Chen L, Shao Y, Luo Q, Deng X, Wu M et al (2018) MicroRNA-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic T2DM rats. Front Pharmacol 9:478
Li H-H, Lin S-L, Huang C-N, Lu FJ, Chiu PY, Huang WN, Lai TJ, Lin CL (2016) miR-302 attenuates amyloid-β-induced neurotoxicity through activation of Akt signaling. J Alzheimers Dis 50:1083–1098
Sørensen SS, Nygaard A-B, Christensen T (2016) miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia—an exploratory study. Translational Neurodegeneration 5
Sun L-L, Jiang B-G, Li W-T, Zou JJ, Shi YQ, Liu ZM (2011) MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract 91:94–100
Hébert SS, Papadopoulou AS, Smith P, Galas MC, Planel E, Silahtaroglu AN, Sergeant N, Buée L et al (2010) Genetic ablation of Dicer in adult forebrain neurons results in abnormal tau hyperphosphorylation and neurodegeneration. Hum Mol Genet 19:3959–3969
Sarkar S, Engler-Chiurazzi EB, Cavendish JZ, Povroznik JM, Russell AE, Quintana DD, Mathers PH, Simpkins JW (2019) Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer’s disease-like pathology. Brain Res 1721:146327
Shen Y, Xu H, Pan X, et al (2017) miR-34a and miR-125b are upregulated in peripheral blood mononuclear cells from patients with type 2 diabetes mellitus. Experimental and Therapeutic Medicine
Sarkar S, Jun S, Rellick S, Quintana DD, Cavendish JZ, Simpkins JW (2016) Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res 1646:139–151
Kim W, Noh H, Lee Y, Jeon J, Shanmugavadivu A, McPhie DL, Kim KS, Cohen BM et al (2016) MiR-126 regulates growth factor activities and vulnerability to toxic insult in neurons. Mol Neurobiol 53:95–108
Fang S, Ma X, Guo S, Lu J (2017) MicroRNA-126 inhibits cell viability and invasion in a diabetic retinopathy model via targeting IRS-1. Oncol Lett 14:4311–4318
Fan D, Yang S, Han Y, Zhang R, Yang L (2020) Isoflurane-induced expression of miR-140-5p aggravates neurotoxicity in diabetic rats by targeting SNX12. J Toxicol Sci 45:69–76
Kalani A, Chaturvedi P, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2017) Dementia-like pathology in type-2 diabetes: a novel microRNA mechanism. Mol Cell Neurosci 80:58–65
Lin X, Qin Y, Jia J, Lin T, Lin X, Chen L, Zeng H, Han Y et al (2016) MiR-155 enhances insulin sensitivity by coordinated regulation of multiple genes in mice. PLoS Genet 12:e1006308
Kou X, Chen D, Chen N (2020) The regulation of microRNAs in Alzheimer’s disease. Front Neurol 11:288
Rodriguez-Ortiz CJ, Prieto GA, Martini AC, et al (2020) miR-181a negatively modulates synaptic plasticity in hippocampal cultures and its inhibition rescues memory deficits in a mouse model of Alzheimer’s disease. Aging Cell 19
Du X, Yang Y, Xu C et al (2017) Upregulation of miR-181a impairs hepatic glucose and lipid homeostasis. Oncotarget 8:91362–91378
Esguerra JLS, Bolmeson C, Cilio CM, Eliasson L (2011) Differential glucose-regulation of microRNAs in pancreatic islets of non-obese type 2 diabetes model Goto-Kakizaki rat. PLoS One 6:e18613
Hadar A, Milanesi E, Walczak M, Puzianowska-Kuźnicka M, Kuźnicki J, Squassina A, Niola P, Chillotti C et al (2018) SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s disease. Sci Rep 8:8465
Thangavel N, Al Bratty M, Akhtar Javed S et al (2017) Targeting peroxisome proliferator-activated receptors using thiazolidinediones: strategy for design of novel antidiabetic drugs. Int J Med Chem 2017:1069718
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2011) Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 32:1626–1633
Tzimopoulou S, Cunningham VJ, Nichols TE et al (2010) A multi-center randomized proof-of-concept clinical trial applying [18F]FDG-PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer’s disease. J Alzheimers Dis 22:1241–1256
Koenig AM (2016) P1-031: effects of the insulin sensitizer metformin on Alzheimer’s disease biomarkers: results of a brief crossover pilot study. Alzheimers Dement 12:P413–P413
Wang Y, An H, Liu T et al (2019) Metformin improves mitochondrial respiratory activity through activation of AMPK. Cell Rep 29:1511–1523.e5
Sacks J, Mulya A, Fealy CE et al (2018) Effect of metformin on mitochondrial pathways in human skeletal muscle cells. Diabetes 67:157–OR
Isik AT, Soysal P, Yay A, Usarel C (2017) The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer’s disease. Diabetes Res Clin Pract 123:192–198
Prakash S, Rai U, Uniyal A, Tiwari V, Singh S (2020) Sitagliptin mitigates oxidative stress and up-regulates mitochondrial biogenesis markers in Brown adipose tissues of high-fat diet fed obese mice through AMPK phosphorylation. Obesity Medicine 19:100265
Panagaki T, Michael M, Hölscher C (2017) Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci Rep 7:16158
Wang R-F, Xue G-F, Hölscher C, Tian MJ, Feng P, Zheng JY, Li DF (2018) Post-treatment with the GLP-1 analogue liraglutide alleviate chronic inflammation and mitochondrial stress induced by Status epilepticus. Epilepsy Res 142:45–52
Moreira P, Santos M, Sena C et al (2005) CoQ10 therapy attenuates amyloid β-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp Neurol 196:112–119
Hsu C-C, Wahlqvist ML, Lee M-S, Tsai H-N (2011) Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 24:485–493
Cardoso S, Santos MS, Seiça R, Moreira PI (2010) Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim Biophys Acta (BBA) - Mol Basis Dis 1802:942–951
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705
Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Plácido A, Santos MS et al (2013) Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta 1832:527–541
Park K-A, Jin Z, Lee JY et al (2020) Long-lasting exendin-4 fusion protein improves memory deficits in high-fat diet/streptozotocin-induced diabetic mice. Pharmaceutics 12. https://doi.org/10.3390/pharmaceutics12020159
Duarte AI, Candeias E, Alves IN, Mena D, Silva DF, Machado NJ, Campos EJ, Santos MS et al (2020) Liraglutide protects against brain amyloid-β1–42 accumulation in female mice with early Alzheimer’s disease-like pathology by partially rescuing oxidative/nitrosative stress and inflammation. Int J Mol Sci 21:1746
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B et al (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20(Suppl 2):S609–S631
Escribano-Lopez I, Bañuls C, Diaz-Morales N, Iannantuoni F, Rovira-Llopis S, Gomis R, Rocha M, Hernandez-Mijares A et al (2019) The mitochondria-targeted antioxidant MitoQ modulates mitochondrial function and endoplasmic reticulum stress in pancreatic β cells exposed to hyperglycaemia. Cell Physiol Biochem 52:186–197
Escribano-López I, de Marañon AM, Iannantuoni F, et al (2019) The mitochondrial antioxidant SS-31 modulates oxidative stress, endoplasmic reticulum stress, and autophagy in type 2 diabetes. J Clin Med Res 8 https://doi.org/10.3390/jcm8091322
Ola MS, Aleisa AM, Al-Rejaie SS et al (2014) Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 35:1003–1008
Grizzanti J, Corrigan R, Casadesus G (2018) Neuroprotective effects of amylin analogues on Alzheimer’s disease pathogenesis and cognition. J Alzheimers Dis 66:11–23
Adler BL, Yarchoan M, Hwang HM, Louneva N, Blair JA, Palm R, Smith MA, Lee HG et al (2014) Neuroprotective effects of the amylin analogue pramlintide on Alzheimer’s disease pathogenesis and cognition. Neurobiol Aging 35:793–801
Abramova NA, Cassarino DS, Khan SM, Painter TW, Bennett JP (2002) Inhibition by R( ) or S(-) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. J Neurosci Res 67:494–500
Harashima S-I, Nishimura A, Osugi T, Wang Y, Liu Y, Takayama H, Inagaki N (2016) Restless legs syndrome in patients with type 2 diabetes: effectiveness of pramipexole therapy. BMJ Support Palliat Care 6:89–93
Sanz-Blasco S, Valero RA, Rodríguez-Crespo I, Villalobos C, Núñez L (2008) Mitochondrial Ca2 overload underlies Aβ oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One 3:e2718
Bellucci PN, González Bagnes MF, Di Girolamo G, González CD (2017) Potential effects of nonsteroidal anti-inflammatory drugs in the prevention and treatment of type 2 diabetes mellitus. J Pharm Pract 30:549–556
Ahn H, Kang SG, Yoon S-I, Ko HJ, Kim PH, Hong EJ, An BS, Lee E et al (2017) Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci Rep 7:12409
Grohm J, Kim S-W, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19:1446–1458
Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT et al (2008) Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell 14:193–204
Asalla S, Girada SB, Kuna RS, Chowdhury D, Kandagatla B, Oruganti S, Bhadra U, Bhadra MP et al (2016) Restoring mitochondrial function: a small molecule-mediated approach to enhance glucose stimulated insulin secretion in cholesterol accumulated pancreatic beta cells. Sci Rep 6:27513
Wang D, Wang J, Bonamy GMC, Meeusen S, Brusch RG, Turk C, Yang P, Schultz PG (2012) A small molecule promotes mitochondrial fusion in mammalian cells. Angew Chem Int Ed Eng 51:9302–9305
Moreira PI (2018) Sweet mitochondria: a shortcut to Alzheimer’s disease. J Alzheimers Dis 62:1391–1401
Availability of Data and Materials
The datasets supporting the conclusions of this article are included within the article.
Funding
We acknowledge the Council of Scientific & Industrial Research (CSIR), India and Science and Engineering Research Board (SERB), India for funding this work. SP acknowledges a junior research fellowship from UGC
Author information
Authors and Affiliations
Contributions
BK conceived the original idea and designed the outlines of the study. SP and DS wrote the draft of the manuscript. SP and DS prepared the figures for the manuscript. SP and DS performed the literature review. SP aided in revising the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent to Publish
NA.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paul, S., Saha, D. & BK, B. Mitochondrial Dysfunction and Mitophagy Closely Cooperate in Neurological Deficits Associated with Alzheimer’s Disease and Type 2 Diabetes. Mol Neurobiol 58, 3677–3691 (2021). https://doi.org/10.1007/s12035-021-02365-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02365-2