Abstract
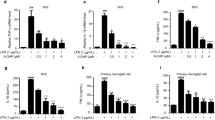
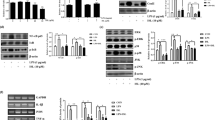
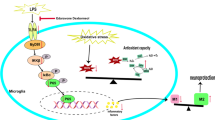
Microglia play a crucial role in the inflammatory brain response to infection. However, overactivation of microglia is neurotoxic. Toll-like receptor 4 (TLR4) is involved in microglial activation via lipopolysaccharide (LPS), which triggers a variety of cytotoxic pro-inflammatory markers that produce deleterious effects on neuronal cells. Ferulic acid (FA) is a phenolic compound that exerts antioxidant and anti-inflammatory effects in neurodegenerative disease. However, the manner in which FA inhibits neuroinflammation-induced neurodegeneration is poorly understood. Therefore, we investigated the anti-inflammatory effects of FA against LPS-induced neuroinflammation in the mouse brain. First, we provide evidence that FA interferes with TLR4 interaction sites, which are required for the activation of microglia-induced neuroinflammation, and further examined the potential mechanism of its neuroprotective effects in the mouse hippocampus using molecular docking simulation and immunoblot analysis. Our results indicated that FA treatment inhibited glial cell activation, p-JNK, p-NFKB, and downstream signaling molecules, such as iNOS, COX-2, TNF-α, and IL-1β, in the mouse hippocampus and BV2 microglial cells. FA treatment strongly inhibited mitochondrial apoptotic signaling molecules, such as Bax, cytochrome C, caspase-3, and PARP-1, and reversed deregulated synaptic proteins, including PSD-95, synaptophysin, SNAP-25, and SNAP-23, and synaptic dysfunction in LPS-treated mice. These findings demonstrated that FA treatment interfered with the TLR4/MD2 complex binding site, which is crucial for evoking neuroinflammation via microglia activation and inhibited NFKB likely via a JNK-dependent mechanism, which suggests a therapeutic implication for neuroinflammation-induced neurodegeneration.









Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- PBS:
-
Phosphate-buffered saline
- NFKB:
-
Nuclear factor kappa B
- TNF-α:
-
Tumor necrosis factor-alpha
- iNOS:
-
Inducible nitric oxide synthase
- COX-2:
-
Cyclooxygenase-2
- p-JNK:
-
Phospho-C-jun N-terminal Kinase
- PARP-1:
-
Poly[ADP-ribose] polymerase 1
- TRITC:
-
Tetramethylrhodamine isothiocyanate
- FITC:
-
Fluorescein isothiocyanate
- DG:
-
Dentate gyrus
- CA1:
-
Cornu Ammonis
- PSD-95:
-
Post-synaptic density-95
- SNAP23:
-
Synaptosomal-associated protein 23
- SD:
-
Standard deviation
- i.p.:
-
Intraperitoneally
- NAOH:
-
Sodium hydroxide
- IKK:
-
IkB kinases
- Iba-1:
-
Ionized calcium-binding adapter molecule 1
- GFAP:
-
Glial fibrillary acidic protein
- KMNO4:
-
Potassium permanganate
References
Jha MK, Jeon S, Suk K (2012) Glia as a link between neuroinflammation and neuropathic pain. Immune Netw 12(2):41–47
Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH, Kim HM, Lee K, Cho WG et al (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflammation 9:35. https://doi.org/10.1186/1742-2094-9-35
Dohi K, Ohtaki H, Nakamachi T, Yofu S, Satoh K, Miyamoto K, Song D, Tsunawaki S et al (2010) Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J Neuroinflammation 7:41. https://doi.org/10.1186/1742-2094-7-41
Wang X, Wang C, Wang J, Zhao S, Zhang K, Wang J, Zhang W, Wu C et al (2014) Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-kappaB, MAPKs and Akt signaling pathways. Neuropharmacology 79:642–656. https://doi.org/10.1016/j.neuropharm.2014.01.022
Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T et al (2016) Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflammation 13(1):77. https://doi.org/10.1186/s12974-016-0541-7
Carrasco E, Casper D, Werner P (2007) PGE(2) receptor EP1 renders dopaminergic neurons selectively vulnerable to low-level oxidative stress and direct PGE(2) neurotoxicity. J Neurosci Res 85(14):3109–3117
Jara JH, Singh BB, Floden AM, Combs CK (2007) Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK-dependent death. J Neurochem 100(5):1407–1420
Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ et al (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22(7):2478–2486
Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on toll-like receptor 4. J Exp Med 189(11):1777–1782
Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, Jung IC, Choi YH (2007) Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-kappaB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol 37(3):1402–1408. https://doi.org/10.1016/j.jep.2011.08.008
Xie Z, Smith CJ, Van Eldik LJ (2004) Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia 45(2):170–179
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934. https://doi.org/10.1016/j.cell.2010.02.016
Mrak RE, Griffin WS (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 26(3):349–354
Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D (2007) Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol 82:277–296
Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25(2):181–213
Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ (2011) Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta). J Neuroinflammation 8:79
Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R (2010) Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. J Neuroinflammation 7:30. https://doi.org/10.1186/1742-2094-7-30
Choi Y, Lee MK, Lim SY, Sung SH, Kim YC (2009) Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1beta by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells. Br J Pharmacol 156(6):933–940. https://doi.org/10.1111/j.1476-5381.2009.00022.x
Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS (2010) Glycogen synthase kinase-3beta inactivation inhibits tumor necrosis factor-alpha production in microglia by modulating nuclear factor kappaB and MLK3/JNK signaling cascades. J Neuroinflammation 7:99. https://doi.org/10.1186/1742-2094-7-99
Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO (2006) Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol 542(1–3):1–7
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69
Zeng KW, Fu H, Liu GX, Wang XM (2010) Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int Immunopharmacol 10(6):668–678. https://doi.org/10.1016/j.intimp.2010.03.010
Barone E, Calabrese V, Mancuso C (2009) Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 10(2):97–108
Zhao Z, Moghadasian MH (2008) Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: a review. Food Chem 109(4):691–702. https://doi.org/10.1016/j.foodchem.2008.02.039
Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL (2008) Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res 1209:136–150
Kim HS, Cho JY, Kim DH, Yan JJ, Lee HK, Suh HW, Song DK (2004) Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull 27:120–121
Sultana R (2012) Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochim Biophys Acta 1822(5):748–752. https://doi.org/10.1016/j.bbadis.2011.10.015
Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH et al (2001) Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol 133(1):89–96
Shah SA, Yoon GH, Chung SS, Abid MN, Kim TH, Lee HY, Kim MO (2017) Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol Psychiatry 22(3):407–416
Shah SA, Amin FU, Khan M, Abid MN, Rehman SU, Kim TH, Kim MW, Kim MO (2016) Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J Neuroinflammation 13(1):286
Rehman SU, Shah SA, Ali T, Chung JI, Kim MO (2016) Anthocyanins reversed D-galactose-induced oxidative stress and Neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol 54(1):255–271
Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD (2003) Improved protein-ligand docking using GOLD. Proteins 52(4):609–623
Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N et al (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130(5):906–917
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acid Res 28(1):235–242
Park BS, Song DH, Kim BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195
Han J., Kim H. J., Lee S. C., Hong S., Park K., Jeon Y. H., Kim D., Cheong H. K., Kim H. S Structure-based rational design of a Toll-like receptor 4 (TLR4) decoy receptor with high binding affinity for a target protein. PloS ONE 7(2):e30929.
Li Y, Han L, Liu Z, Wang R (2014) Comparative assessment of scoring functions on an updated benchmark: 2. Evaluation methods and general results. J Chem Inf Model 54(6):1717–1736. https://doi.org/10.1021/ci500081m
Ni H, Zhao W, Kong X, Li H, Ouyang J (2014) Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol 131(2):102–111
Matzinger P (2002) The danger model: a renewed sense of self. Science 296(5566):301–305
Svajger U, Brus B, Turk S, Sova M, Hodnik V, Anderluh G, Gobec S (2013) Novel toll-like receptor 4 (TLR4) antagonists identified by structure- and ligand-based virtual screening. Eur J Med Chem 70:393–399. https://doi.org/10.1016/j.ejmech.2013.10.019
Verstak B, Nagpal K, Bottomley SP, Golenbock DT, Hertzog PJ, Mansell A (2009) MyD88 adapter-like (mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J Biol Chem 284(36):24192–24203
Zhang L, Wu C, Zhao S, Yuan D, Lian G, Wang X, Wang L, Yang J (2010) Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol 10(3):331–338. https://doi.org/10.1016/j.intimp.2009.12.004
Roy A, Jana A, Yatish K, Freidt MB, Fung YK, Martinson JA, Pahan K (2008) Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: implications for neurodegenerative diseases. Free Radic Biol Med 45(5):686–699
Debatin KM, Poncet D, Kroemer G (2002) Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene 21(57):8786–8803
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41(2–3):242–247
Li D, Li X, Wu J, Li J, Zhang L, Xiong T, Tang J, Qu Y et al (2015) Involvement of the JNK/FOXO3a/Bim pathway in neuronal apoptosis after hypoxic-ischemic brain damage in neonatal rats. PLoS One 10(7):e0132998. https://doi.org/10.1371/journal.pone.0132998
Fruhauf PK, Ineu RP, Tomazi L, Duarte T, Mello CF, Rubin MA (2015) Spermine reverses lipopolysaccharide-induced memory deficit in mice. J Neuroinflammation 12:3. https://doi.org/10.1186/s12974-014-0220-5
Nakajima K, Kohsaka S (1998) Functional roles of microglia in the central nervous system. Hum Cell 11(3):141–155
Czerniawski J, Miyashita T, Lewandowski G, Guzowski JF (2015) Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain Behav Immun 44:159–166. https://doi.org/10.1016/j.bbi.2014.09.014
Gabellec MM, Griffais R, Fillion G, Haour F (1995) Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res 31(1–2):122–130
Badshah H, Ali T, Rehman S-U, Faiz-ul A, Ullah F, Kim TH, Kim MO (2016) Protective effect of lupeol against lipopolysaccharide-induced neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol 11:48–60
Agostinho P, Cunha RA, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16(25):2766–2778
Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL, Liu YD et al (2011) Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS One 6(7):e21891. https://doi.org/10.1371/journal.pone.0021891
Aravalli RN, Peterson PK, Lokensgard JR (2007) Toll-like receptors in defense and damage of the central nervous system. J NeuroImmune Pharmacol 2(4):297–312
Yoon HM, Jang KJ, Han MS, Jeong JW, Kim GY, Lee JH, Choi YH (2013) Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-kappaB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells. Exp Ther Med 5(3):957–963
Doyle SL, O'Neill LA (2006) Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol 72(9):1102–1113
Liu S, Kielian T (2009) Microglial activation by Citrobacter koseri is mediated by TLR4- and MyD88-dependent pathways. J Immunol 183(9):5537–5547. https://doi.org/10.4049/jimmunol.0900083
Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T (2013) Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS One 8(2):e55774. https://doi.org/10.1371/journal.pone.0055774
Kuper C, Beck FX, Neuhofer W (2012) Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol 302(1):F38–F46. https://doi.org/10.1152/ajprenal.00590.2010
Perkins ND (2007) Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8(1):49–62
Lampiasi N, Montana G (2016) The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 221(3):486–493
Koshiguchi M, Komazaki H, Hirai S, Egashira Y (2017) Ferulic acid suppresses expression of tryptophan metabolic key enzyme indoleamine 2, 3-dioxygenase via NFκB and p38 MAPK in lipopolysaccharide-stimulated microglial cells. Biosci Biotechnol Biochem 81(5):966–971. https://doi.org/10.1080/09168451.2016.1274636
Gatheral T, Reed DM, Moreno L, Gough PJ, Votta BJ, Sehon CA, Rickard DJ, Bertin J et al (2015) A key role for the endothelium in NOD1 mediated vascular inflammation: comparison to TLR4 responses. PLoS One 7(8):e42386. https://doi.org/10.1371/journal.pone.0042386
Kim IT, Ryu S, Shin JS, Choi JH, Park HJ, Lee KT (2012) Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-κB inactivation in RAW 264.7 macrophages. J Cell Biochem 113(6):1936–1946. https://doi.org/10.1002/jcb.24062
Nikseresht S, Khodagholi F, Nategh M, Dargahi L (2015) RIP1 inhibition rescues from LPS-induced RIP3-mediated programmed cell death, distributed energy metabolism and spatial memory impairment. J Mol Neurosci 57(2):219–230. https://doi.org/10.1007/s12031-015-0609-3
Santhanasabapathy R, Sudhandiran G (2015) Farnesol attenuates lipopolysaccharide-induced neurodegeneration in Swiss albino mice by regulating intrinsic apoptotic cascade. Brain Res 1620:42–56. https://doi.org/10.1016/j.brainres.2015.04.043
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11(10):2481–2504. https://doi.org/10.1089/ARS.2009.2578
Pan XD, Chen XC, Zhu YG, Zhang J, Huang TW, Chen LM, Ye QY, Huang HP (2008) Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochem Pharmacol 76(3):362–372. https://doi.org/10.1016/j.bcp.2008.05.018
Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R (2003) P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem 278(15):13309–13317
Catino S, Paciello F, Miceli F, Rolesi R, Troiani D, Calabrese V, Santangelo R, Mancuso C (2015) Ferulic acid regulates the Nrf2/heme oxygenase-1 system and counteracts trimethyltin-induced neuronal damage in the human neuroblastoma cell line SH-SY5Y. Front Pharmacol 6:305. https://doi.org/10.3389/fphar.2015.00305
Shen Y, Zhang H, Wang L, Qian H, Qi Y, Miao X, Cheng L, Qi X (2016) Protective effect of ferulic acid against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidative stress in PC12 cells. Cell Mol Biol (Noisy-le-grand) 62(1):109–116
Tsai FS, Wu LY, Yang SE, Cheng HY, Tsai CC, Wu CR, Lin LW (2015) Ferulic acid reverses the cognitive dysfunction caused by amyloid beta peptide 1-40 through anti-oxidant activity and cholinergic activation in rats. Am J Chin Med 43(2):319–335. https://doi.org/10.1142/S0192415X15500214
Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF (2015) Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289:429–442. https://doi.org/10.1016/j.neuroscience.2015.01.001
Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M (2010) Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 107(47):20518–20522. https://doi.org/10.1073/pnas.1014557107
Acknowledgments
This research work was supported by the Brain Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT (2016M3C7A1904391).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All the experiments and animal experimental procedures were approved (Approval ID 125) by the animal ethics committee (IACUC) of the Division of Life Science, Department of Biology, Gyeongsang National University, Republic of South Korea. All the animals were carefully handled and acclimatized for 2 weeks before starting the experimental procedure as according to the animal ethics committee (IACUC) of the Division of Life Science, Department of Biology, Gyeongsang National University, Republic of South Korea.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 633 kb)
Rights and permissions
About this article
Cite this article
Rehman, S.U., Ali, T., Alam, S.I. et al. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol Neurobiol 56, 2774–2790 (2019). https://doi.org/10.1007/s12035-018-1280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1280-9