Abstract
Members of the Toll-like receptor (TLR) family play critical roles as regulators of innate and adaptive immune responses. TLRs function by recognizing diverse molecular patterns on the surface of invading pathogens. In the brain, microglial cells generate neuroimmune responses through production of proinflammatory mediators. The upregulation of cytokines and chemokines in response to microbial products and other stimuli has both beneficial and deleterious effects. Emerging evidence demonstrates a central role for TLRs expressed on microglia as a pivotal factor in generating these neuroimmune responses. Therefore, understanding the basis of TLR signaling in producing these responses may provide insights into how activated microglia attempt to strike a balance between defense against invading pathogens and inflicting irreparable brain damage. These insights may lead to innovative therapies for CNS infections and neuroinflammatory diseases based on the modulation of microglial cell activation through TLR signaling.
Similar content being viewed by others
Introduction
The mammalian immune system uses a number of mechanisms to successfully detect and eliminate invading pathogens, many of which require the discrimination between self and nonself. Immune responses have historically been divided into two distinct components: innate immunity and adaptive immunity. The innate immune response, which is highly conserved in most multicellular organisms, provides the first line of defense to limit infection and detect pathogens using germline-encoded proteins (Hoffmann et al. 1999). Adaptive immunity, which is present only in vertebrates, can detect nonself through the recognition of peptide antigens using receptors expressed on the surface of B and T cells (Akira and Takeda 2004). Adaptive responses are much more diverse than innate responses in that each B and T lymphocyte clone expresses a distinct antigen receptor, which arose through somatic gene rearrangement (Fearon 1997). Although extensive studies have produced a wealth of information regarding adaptive immune responses, the innate mechanisms of immunity are just beginning to be understood.
Innate immune responses emanate from interactions between host-cell surface receptors and conserved structural motifs, termed pathogen-associated molecular patterns (PAMP) on the surface of pathogens. There are a number of key receptors that recognize a variety of PAMPs including toll-like receptors (TLR), NOD-like receptors (NLR), and RIG-I-like receptors (RLR) (Creagh and O’Neill 2006). All of these receptors, either acting alone or in concert with other molecules, generate innate immune responses to counter invading pathogens. Whereas TLR family members recognize bacteria, viruses, fungi, and protozoa, NLRs usually detect bacteria and RLRs recognize viruses. In this review, we discuss recent advances regarding the critical role for TLR signaling during central nervous system (CNS) infections and the damage to the CNS associated with infectious or inflammatory diseases of the brain.
Toll-like receptor signaling in mammalian cells
Toll was first identified as a gene important for the establishment of dorsal–ventral orientation during embryonic development in Drosophila melanogaster (Hashimoto et al. 1988). Later, Toll was shown to play a critical role in Drosophila’s immunity to fungal infections (Lemaitre et al. 1996). In fact, the first mammalian homolog of Toll, Toll-like receptor 4 (TLR4), was identified as a pattern recognition receptor required for adaptive immunity (Medzhitov et al. 1997). Subsequently, TLR4 and its family members were shown to play critical roles in generating innate immune responses to infection in mammalian systems (Table 1). To date, 11 human TLRs and 13 murine TLRs have been identified, which trigger both innate and adaptive immune responses (Akira and Takeda 2004; Kawai and Akira 2006).
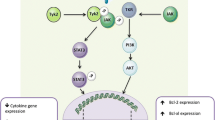
TLRs are membrane-bound proteins that contain varying numbers of highly conserved extracellular leucine-rich repeats (LRR) and a highly conserved Toll-interleukin (IL)-1 receptor (TIR) domain in the cytoplasmic region (Fig. 1). They recognize ligands through their LRRs and transmit signals intracellularly through their TIR domain via protein–protein interactions with adaptor molecules such as MyD88, TIRAP/Mal, TRIF, and TRAM. Cellular adaptor proteins then trigger a cascade of signaling events resulting in the phosphorylation of IRAK and activation of downstream effector molecules such as NF-κB or IRF3, culminating in the production of immune mediators as well as IFN-inducible genes (Akira et al. 2006). Thus, a direct or indirect association of a ligand with its cognate TLR serves as a signal to trigger innate immune responses.
Toll-like receptor signaling pathways. TLRs on cytoplasmic and endosomal membranes recognize a variety of patterns (PAMPs) on pathogens and signal through adaptor molecules (e.g., MyD88, TIRAP/Mal, TRAM, TRIF) via the TIR domain, located on their cytoplasmic tail. Adaptor molecules activate downstream proteins in the signaling cascades leading to release of NF-κB, from its inhibitor IκB. TLR signaling in response to specific stimuli also activate IRF3, IRF5, and IRF7. After activation, these proteins translocate into the nucleus and induce the transcription of proinflammatory immune mediators and IFN-responsive genes.
TLRs are highly conserved and it appears that most cells express low levels of TLRs constitutively in a cell-type specific manner. In a comprehensive molecular phylogenetic analysis, vertebrate TLRs were recently classified into six distinct families based upon amino acid sequence homologies of LRRs (Roach et al. 2005). Intense research efforts during the past few years have resulted in the identification of a large number of molecules involved in TLR activation pathways (Akira and Takeda 2004; Miggin and O’Neill 2006). Every step along these pathways is tightly regulated by a complex mix of phosphorylation, targeted degradation, and sequestration of signaling molecules depending upon the nature of the invading pathogen (Miggin and O’Neill 2006).
TLR polymorphisms and susceptibility to infections
Although the human genome consists of 3 billion base pairs it is highly conserved within the species. However, inter-individual variations do occur in about 0.1% of genomic sequences resulting in approximately 10 million variants or “polymorphisms” in the human genome (Goldstein and Cavalleri 2005). The most common type of such variations is the single nucleotide polymorphism (SNP), where a single-point mutation occurs, causing either an amino acid substitution potentially altering the function of the protein, or formation of a premature stop codon leading to the production of a truncated protein. The importance of SNPs in the susceptibility to common infections, such as tuberculosis, sepsis, malaria, HIV, and pneumonia, has been well established (Schork et al. 2000; Cooke and Hill 2001; Segal and Hill 2003; Frodsham and Hill 2004).
Recent studies suggest that SNPs in TLR genes alter susceptibility to infectious diseases. The first SNP in a TLR gene was reported for TLR4. In a study of 83 healthy individuals subjected to LPS inhalation challenge, two mutations, Asp299Gly and Thr399Ile, were identified in the TLR4 gene that correlated with hyporesponsiveness to LPS. Interestingly, Asp299Gly mutation, but not Thr399Ile, interrupts TLR4-mediated LPS signaling (Arbour et al. 2000). This SNP was also found to increase susceptibility to septic shock caused by Gram-negative bacteria (Lorenz et al. 2002) and was suggested to have a protective role in atherosclerosis (Kiechl et al. 2002). In a case-control study conducted among 870 Ghanaian children, both these SNPs were found to occur at higher rates rendering them susceptible to malaria resulting from Plasmodium falciparum infection (Mockenhaupt et al. 2006). In another study involving 197 patients with meningococcal disease caused by the organism Neiserria meningiditis in the United Kingdom, 11 other rare missense mutations in the TLR4 gene were identified (Smirnova et al. 2003). Whereas TLR4 SNPs render an individual susceptible to some infectious diseases, they were also found to have no effect on the likelihood or severity of others such as meningococcal disease (Read et al. 2001). Further studies on the functional relevance of these variants will provide information on their contribution to disease susceptibility.
To date, two SNPs, Arg753Gln and Arg677Trp, have been described for TLR2. The Arg753Gln polymorphism, originally described in patients with leprosy (Kang and Chae 2001) also confers susceptibility to tuberculosis (Ogus et al. 2004; Schroder et al. 2005). Interestingly, this SNP was demonstrated to have a protective effect from late-stage Lyme disease caused by Borrelia burgdorferi infection (Schroder et al. 2005), and this protective effect could be due to impaired immune responses caused by reduced TLR2 signaling. Recently, the Arg677Trp SNP has been identified as an artifact resulting from a TLR2 pseudogene (Malhotra et al. 2005). This finding underlines the importance of using appropriate molecular procedures in determining the SNP data and validating the results using different methodologies.
Although the SNPs normally occur in exons, a guanine-thymine (GT) repeat polymorphism was recently observed in the intron II of TLR2 gene. In a study of 174 Korean patients with tuberculosis, shorter GT repeats (“microsatellites”) were found to be a very common occurrence. These GT repeats resulted in lower TLR2 expression and caused increased susceptibility to tuberculosis (Yim et al. 2006a). Incidentally, these microsatellites in the TLR2 gene were found to be highly conserved as shown for 12 different nonhuman primates (Yim et al. 2006b). Frequent occurrence of SNPs in TLR genes lead to abnormal immune responses and could thereby lead to high incidence of infectious diseases. Therefore, understanding how such genetic variations in key innate immune genes alter disease susceptibility is critical in developing improved diagnosis and new therapies.
Endogenous ligands for Toll-like receptors
In addition to the PAMPs, which are contained within or released from the cell wall of extracellular pathogens such as lipopolysaccharide (LPS), the major cell wall component of Gram-negative bacteria, and peptidoglycan, the major cell wall component of Gram-positive bacteria, a number of endogenous ligands have been identified for TLRs, including heat shock proteins (hsp), mRNA, high mobility group box 1 (HMGB1) protein, surfactant proteins A and D (SP-A and SP-D), hyaluronan, and fibrinogen (Smiley et al. 2001; Asea et al. 2002; Bulut et al. 2002; Guillot et al. 2002; Termeer et al. 2002; Kariko et al. 2004; Park et al. 2004; Zhou et al. 2005; Ohya et al. 2006; Scheibner et al. 2006). Various products from necrotic cells such as polysaccharide fragments of hyaluronan and heparin sulfate also activate TLRs. These molecules are released from damaged, dead or ruptured cells in response to tissue damage, injury, and infection (Johnson et al. 2003). Interestingly, Schwann cells of the peripheral nervous system also trigger the production of inflammatory cytokines in response to necrotic neuronal cells via TLR signaling (Lee et al. 2006). Activated Schwann cells clear the neuronal cell and myelin debris by phagocytosis and pave the way for nerve repairing processes to take place (Stoll et al. 2002). During Wallerian nerve degeneration, activated Schwann cells from wild-type mice produced tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS) via TLR2 and TLR3 induction, whereas Schwann cells derived from TLR2−/− and TLR3−/− animals did not produce these immune mediators (Lee et al. 2006).
Heat shock proteins serve as prime examples of endogenous ligands of TLR signaling. Using complementation studies with macrophages isolated from C3H/HeJ mice that lack TLR4 gene expression, Hsp60 was demonstrated to function as a ligand for TLR4 and TLR4 stimulation by Hsp60-induced production of cytokines (Ohashi et al. 2000). Following this observation, heat shock proteins Hsp60, Hsp70, and Hsp90 from both bacterial and mammalian origin, as well as their endoplasmic reticulum form gp96, were shown to trigger the production of proinflammatory immune mediators such as TNF-α, interleukin (IL)-1β, IL-6, and IL-8 by macrophages, endothelial cells, dendritic cells, and monocytes. Release of nitric oxide (NO) and chemokines by these cells has also been reported (Ohashi et al. 2000; Bulut et al. 2002; Vabulas et al. 2002a, b; Asea et al. 2002; Bandholtz et al. 2003). Similar complementation studies have been conducted with TLR4−/− (C3H/HeJ or C57BL/10ScCr) mice to demonstrate that β-defensin 2, SP-A, heparin sulfate, fibronectin A, hyaluronan, and fibrinogen also could function as endogenous TLR4 ligands (Okamura et al. 2001; Smiley et al. 2001; Biragyn et al. 2002; Guillot et al. 2002; Johnson et al. 2002; Termeer et al. 2002; Vabulas et al. 2002a, b). Recently, hyaluronan was shown to function as a “danger signal” and activate TLR2 (Scheibner et al. 2006). Other studies conducted along similar lines demonstrated that heat shock proteins and HMGB1 act as ligands for both TLR2 and TLR4 (Vabulas et al. 2002a, b; Park et al. 2004; Yu et al. 2006). Another ligand, the epithelial cell-derived inhibitor of leukocyte serine proteases (SLPI) suppresses LPS-induced production of TNF-α and the activation of NF-κB (Jin et al. 1997).
While the identification of endogenous ligands for TLRs has advanced knowledge of the mechanisms, as well as the complexity, of innate immunity, these studies also generated controversy because a number of experiments describing these mechanisms, especially with hsp, were performed using proteins that were later found to be contaminated with LPS (endotoxin) (Majde 1993; Gao et al. 2001; Reed et al. 2003; Tsan and Gao 2004). Apart from LPS, many commercially available peptides have also been found to be contaminated with other microbial cell wall components such as peptidoglycan (PGN) (Majde 1993). For these studies, it is vital to ensure that there is no LPS contamination in the preparations being tested, as even a small quantity of LPS can be a potent inducer of proinflammatory molecules. It is not clear whether the results obtained for other putative endogenous ligands reported were caused by LPS contamination. Significantly, a recent study showed that the expression of Hsp70 in monocytes is a downstream event that occurs after the induction of TLR2 and TLR4, suggesting that Hsp70 does not activate TLRs (Zhou et al. 2005). In the light of these findings, it is imperative to re-evaluate the conclusions of such earlier studies.
Toll-like receptor expression in brain cells
The cell types found within the brain are highly conserved between different species of vertebrates. In addition to neurons, the CNS is comprised of three glial cell populations: astrocytes and oligodendrocytes, which, like neurons are of neuroectodermal origin, and microglia, which are derived from mesoderm. A major function of all three glial cell types is to provide support for neurons. Because a large number of CNS infections are caused by pathogens known to activate TLR signaling in a variety of different cell types (Table 1), it can be expected that brain cells also constitutively express TLRs. Of all four cell types in the CNS, not surprisingly, the microglial cell, the key innate immune cell of the brain, expresses all known TLRs. In fact, microglia have been reported to express mRNAs for TLRs 1–9 (Lee and Lee 2002; Olson and Miller 2004; Jack et al. 2005; McKimmie and Fazakerley 2005; Jung et al. 2005), whereas neurons (Prehaud et al. 2005) and oligodendrocytes (Bsibsi et al. 2002) express only mRNA for TLR3.
Experiments with astrocytes, however, have produced inconsistent results. Using highly purified (>99% pure) human astrocytes, work in our laboratory has shown that LPS does not stimulate cytokine and chemokine release and that TLR4 mRNA is undetectable in these brain cells. However, another study of human astrocytes demonstrated the expression of TLR2 and TLR4 mRNA in these cells (Carpentier et al. 2005), whereas the expression of mRNAs for TLRs 1 to 10, with the exception of TLR8, was reported for murine astrocytes (Lee and Lee 2002). However, caution regarding the interpretation of these reports is in order as the presence of a small number of microglial cells within the astrocyte cultures could be mistakenly attributed to astrocytes. Also, achieving astrocyte cultures of >99% homogeneity has proven especially difficult in the murine system. Furthermore, the expression of TLRs at the protein level remains to be demonstrated for astrocytes.
To date, specific ligands for TLRs 1 and 6 have not been identified, and it is believed that they do not function as separate entities. Both these TLRs associate with TLR2 to form heterodimers, which are involved in PAMP recognition. Although TLR2 can function as a homodimer on its own, its association with TLR1 and TLR6 increases the range of substrates it can recognize and thus, among the TLR family members, it is unique in possessing multiple functions. TLR2 associates with TLR1 and TLR6 to recognize triacylated and diacylated lipoproteins, respectively (Akira and Takeda 2004; Kawai and Akira 2006; Miggin and O’Neill 2006). The expression of TLR2 on microglial cells and its recognition of various PAMPs has been well-established during the past few years (Laflamme et al. 2001; Bsibsi et al. 2002; Rasley et al. 2002; Zekki et al. 2002; Laflamme et al. 2003; Olson and Miller 2004; Aravalli et al. 2005; Kielian et al. 2005). In a recent study using TLR2−/− astrocytes, it was reported that TLR2 signaling is essential in enabling astrocytes to recognize PGN from Staphylococcus aureus, as well as for recognition of the intact bacterium itself (Kielian et al. 2005). In contrast, microglial cells do not recognize intact staphylococci, but they can recognize PGN.
Another remarkable feature of TLR2 is its ability to cooperate with CD14 and myeloid differentiation protein (MD)-2 on the host cell surface (Becher et al. 1996; Dziarski et al. 2001; Dobrovolskaia and Vogel 2002). TLR1/2 and TLR2/6 heterodimers cooperate with CD14 to recognize Gram-positive bacterial PAMPs (Cleveland et al. 1996; Dziarski et al. 1996; Gupta et al. 1996; Henneke et al. 2001; Schroder et al. 2003; Weber et al. 2003; Manukyan et al. 2005). This cooperation is also necessary for the recognition of S. aureus PGN (Esen and Kielian 2005). The prime example of the coordination between these two receptors was recently demonstrated with group B Streptococcus (GBS) (Medvedev et al. 1998; Henneke et al. 2001; Park et al. 2004; Lehnardt et al. 2006).
Similar findings were reported using murine macrophages exposed to heat-killed GBS. In TLR2−/− macrophages, production of TNF-α took place normally indicating that it is not dependent upon TLR2 expression. However, the induction of IL-1β, IL-6, and lipocalin 2 occurred only in the presence of TLR2 signaling (Draper et al. 2006). Our recent results with microglial cells infected with herpes simplex virus (HSV)-1 showed that, although a low level of TNF-α production did occur in the absence of TLR2, the production of a large number of key inflammatory cytokines and chemokines was dependent upon TLR2 signaling (Aravalli et al. 2005). Furthermore, the activation of TLR2 signaling on murine microglial cells was found to be required for the induction of microglial cell apoptosis in response to HSV infection (Aravalli et al. 2006).
The association of TLR2 with MD-2 enhances its ability to respond to Gram-negative and Gram-positive bacterial cell wall components and to synthetic LPS (Dziarski and Gupta 2000; Dziarski et al. 2001). Without this association, TLR2 cannot respond to highly purified LPS. Thus, MD-2 functions as a helper molecule for TLR2 (Dziarski et al. 2001). One recent study demonstrated that human oligodendrocytes also express mRNA for TLR2 (Bsibsi et al. 2002). Further experiments are needed to determine conclusively the functional significance of TLR2 in this cell type.
The ligand for TLR3 is dsRNA, an intermediate formed during viral infection, and studies on TLR3 signaling frequently use the synthetic agonist poly(I:C) to mimick dsRNA. A number of studies have shown that both microglial cells and astrocytes express TLR3 mRNA (Bsibsi et al. 2002; Olson and Miller 2004; Carpentier et al. 2005; Farina et al. 2005; Scumpia et al. 2005; Bsibsi et al. 2006). Interestingly, unlike the situation in astrocytes, TLR3 expression in microglia is not regulated by poly(I:C), although they respond to this synthetic agonist by producing IL-1β and IL-6 (Olson and Miller 2004; Kielian et al. 2005). Theiler’s murine encephalomyelitis virus (TMEV) activates TLR3 signaling in microglial cells, as does HIV (Town et al. 2006). In addition to astrocytes and microglia, neurons and oligodendrocytes also express TLR3 (Bsibsi et al. 2002; Prehaud et al. 2005). In studies using astrocytes isolated from TLR3 and MyD88 knockout mice, it has been shown that TLR3 signaling is required for the induction of CCL2/monocyte chemoattractant protein 1 (MCP-1), CXCL10/IP-10, and IL-8 in response to TMEV and coxsackie virus (Park et al. 2006; So et al. 2006). These findings collectively suggest that TLR3 signaling plays a critical role in regulating the outcome of demyelinating diseases, as both TMEV and coxsackie virus induce fatal demyelination.
TLR4 recognition of the Gram-negative cell wall component LPS has been well documented in a variety of cell types, including brain cells. A number of studies have demonstrated the expression of TLR4 by microglia (Laflamme et al. 2001; Bsibsi et al. 2002; Laflamme et al. 2003; Lehnardt et al. 2003; Olson and Miller 2004; Chakravarty and Herkenham 2005; Jung et al. 2005). With the previously mentioned caveat in mind regarding potential contamination of cell cultures with microglia, astrocytes (Bsibsi et al. 2002; Bowman et al. 2003; Carpentier et al. 2005), oligodendrocytes (Lehnardt et al. 2002) and neurons (Wadachi and Hargreaves 2006) have also been reported to express TLR4 mRNA. Microglial cells isolated from TLR4−/− mice do not respond to LPS treatment (Kitamura et al. 2001; Qin et al. 2005; Wang et al. 2005), but if LPS is present in excess, it can trigger immune responses via TLR4-independent mechanisms (Kitamura et al. 2001; Qin et al. 2005). Similar observations were reported with microglial cells from MyD88−/− animals, demonstrating that LPS activation in microglial cells could occur possibly via another pattern recognition receptor (PRR) (Esen and Kielian 2006). Another recent study using CD14−/− microglial cells found that CD14 is required for microglial activation in response to low concentrations of LPS, but not for higher concentrations (>1 μg/ml) (Esen and Kielian 2005). This finding indicates that an interaction with CD14 is not required for TLR4 to recognize LPS. However, an interaction with CD14 does maximize the ability of TLR4 to recognize LPS and to elicit strong immune responses (Dobrovolskaia and Vogel 2002).
TLR4 expression in astrocytes has been controversial. While some groups have identified low levels of TLR4 mRNA expression (Bsibsi et al. 2002; Bowman et al. 2003; Carpentier et al. 2005), others have failed to detect it both in vitro and in vivo (Laflamme et al. 2001; Lehnardt et al. 2002, 2003; Farina et al. 2005). However, the possibility remains that these cells were microglia rather than astroglia. In a recent study using immunohistochemical analysis, it was reported that human and rat trigeminal nociceptive neurons express TLR4 and the costimulatory receptor CD14 (Wadachi and Hargreaves 2006). Based upon this finding, it was suggested that the pain caused by odontogenic infections could be a direct result of the activation of nociceptors by bacterial products such as LPS.
The expression of TLR5 mRNA has been reported for microglia (Bsibsi et al. 2002; Olson and Miller 2004) and astrocytes (Bowman et al. 2003; Carpentier et al. 2005). In response to the TLR5 ligand flagellin from Salmonella typhimurium, microglial cells were found to upregulate TLR2, TLR4, and TLR5, as well as the expression of IL-6 (Bowman et al. 2003). Further studies are needed to establish the importance of TLR5 expression by glial cells.
TLR7 and TLR8 are homologous to each other and recognize the same set of ligands. The synthetic compounds imiquimod and resquimod that function as ligands for TLR7 and TLR8 are currently being used to treat herpes virus infections. The natural ligand for both these receptors is ssRNA (Diebold et al. 2004) and their expression at the mRNA level was reported in microglia (Bsibsi et al. 2002; Olson and Miller 2004) and astrocytes (Carpentier et al. 2005). A recent report further confirmed the expression of TLR7/8 by microglial cells, where systemic injection of rat spinal cord with the TLR7/8 agonist R848 was found to activate these cells (Zhang et al. 2005a). Another report suggested that TLR8 plays a critical role in regulating the activity of CD4+ regulatory T cells (Peng et al. 2005), which may play a role in controlling autoimmune diseases and cancer. During brain development, murine TLR8 is expressed in neurons and functions as a negative regulator of neurite outgrowth (Ma et al. 2006). Upon stimulation with the TLR8 agonist R848, TLR8 signaling induces apoptosis of neuronal cells. Interestingly, during TLR8 induction, IκBα and IRAK4 were found to be downregulated, implying that TLR8 signaling in rat cortical neurons occurs in a NF-κB-independent manner (Ma et al. 2006).
TLR9 recognizes dsDNA, both bacterial and viral, and synthetic oligodeoxynucleotides (ODN) containing CpG motifs. Both microglial cells and astrocytes have been shown to express mRNA for TLR9 and respond to CpG ODN by producing IL-1β, TNF-α, IL-6, IL-12, macrophage inflammatory protein (MIP)-1α and MIP-1β (Takeshita et al. 2001; Dalpke et al. 2002; Bowman et al. 2003; Olson and Miller 2004; Carpentier et al. 2005; Zhang et al. 2005b; Prinz et al. 2006). Additionally, CpG-containing bacterial DNA upregulated iNOS expression in rodent glial cells through a pathway involving MyD88 and p38 MAPK (Hosoi et al. 2004). Activation of the c-jun N-terminal kinase (JNK) pathway was also reported to occur in astrocytes after treatment with CpG ODN (Lee et al. 2004). JNK and NF-κB activation are critical for the production of proinflammatory cytokines and chemokines in these cells. In contrast to rodent astrocytes, human fetal astrocytes have not been shown to express TLR9 (Farina et al. 2005).
It is currently unknown if any brain cell type expresses the other known TLRs. The human homolog of mouse TLR11, which recognizes profiling-like protein of Toxoplasma gondii, is nonfunctional because of the presence of a premature stop codon in its coding sequence (Zhang et al. 2004; Yarovinsky et al. 2005). Human TLR10 has been shown, so far, to be expressed only in B cells, where it forms heterodimers with TLR1 and TLR2 (Hasan et al. 2005). Further studies are needed to evaluate the expression of TLR10 in the brain and its importance in CNS infections.
Expression of TLRs during astroglial and neuronal development relates to the age-specific regulation of antimicrobial responses. So far, TLR8 is the only TLR that has been detected in mice during embryonic and neonatal stages of development (Ma et al. 2006). Age-dependent expression of other TLRs in the brain is largely unknown. Similarly, no data are currently available regarding the expression of TLRs in neural precursor cells.
Significance of TLR signaling in CNS infections and inflammation
Activation of microglial cells occurs in response to TLR signaling and the role of these activated macrophages in brain inflammation has been well documented (Banati et al. 1993; Chao et al. 1993; Mathiesen and Johnson 1997; Townsend and Scheld 1998; Aschner et al. 1999; Honig and Rosenberg 2000; Aloisi 2001; Lee and Lee 2002; Danton and Dietrich 2003; Ekdahl et al. 2003; Rock et al. 2004; Streit et al. 2004; Kim and de Vellis 2005; Pahlman et al. 2006). It is clear that activated microglia produce a number of immune mediators that influence the ability of neurons to process information. As described in this review, neuroinflammation occurs in response to CNS damage and to brain infection, and microglia play an important role in both of these pathological conditions.
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS whose pathogenesis is determined by cell-mediated autoimmunity. In both MS and its animal model counterpart experimental autoimmune encephalitis (EAE), autoaggressive lymphocytes invade the CNS and induce tissue destruction. To prime T cells and maintain an encephalitogenic response in the CNS, competent antigen-presenting cells (APC) such as macrophages and dendritic cells are needed (Paul 1998). Activation and maturation of APCs in response to various PAMPs causes the breakdown of tolerance for the development of autoimmune responses, leading to demyelination and tissue degeneration (Visser et al. 2005).
TLR ligands such as CpG ODN and PGN are commonly used to trigger EAE in animal models. Recent evidence suggests that bacterial PAMPs, such as S. aureus PGN, also cause demyelination by enabling immune-mediated recognition of self antigens (Kielian et al. 2005; Esen and Kielian 2005). Such studies underscore the importance of TLR signaling in modulating neuropathogenesis. Experiments using TLR9−/− and MyD88−/− mice have shown that both this TLR and its adaptor protein are required for generating immune responses in mice treated with CpG ODN. In addition, C57BL/6 mice in which myelin oligodendrocyte glycoprotein peptide (MOG) was administered subcutaneously did not develop EAE, suggesting a role for TLR2 in the innate neuroimmune response during EAE (Visser et al. 2005). Another line of evidence using CpG ODN showed that mice lacking the MyD88 adapter protein were also resistant to EAE (Prinz et al. 2006). Studies with pertussis toxin (PTX) showed that PTX can induce EAE and modulate leukocyte entry into the CNS. Significantly, this leukocyte trafficking was severely restricted in TLR4−/− mice treated with PTX, demonstrating that TLR4-mediated signals are required for EAE induction (Kerfoot et al. 2004). In a viral disease model, TMEV infection of murine CNS results in a demyelinating disease similar to human MS. It was shown that TMEV infection of astrocytes induced production of CCL2/MCP-1 and IL-8 via activation of the TLR3 pathway (So et al. 2006). It was also demonstrated that TMEV-infected astrocytes from TLR3−/− and MyD88−/− mice did not produce these immune mediators.
A large number of different viruses can cause acute meningitis (localized to the meninges and subarachnoid space) or encephalitis (infection within the brain parenchyma). Infection with human cytomegalovirus (HCMV) is common in the fetus and in infants. In primary syncytiotrophoblast cultures, UV-inactivated HCMV triggered the production of proinflammatory cytokines TNF-α and IL-8, and induced apoptosis. However, when treated with neutralizing antibody to TLR2, both cytokine production and apoptosis were inhibited indicating that binding of UV-inactivated virus to TLR2 can initiate inflammation (Chan and Guilbert 2006).
Acute infection of the brain with herpes simplex virus (HSV) can lead to encephalitis. Mortality or survival with significant neurological deficits (cognitive or physical impairments) are common sequelae of untreated herpes encephalitis. In vitro, both human and murine microglial cells respond to HSV-1 by producing a large number of proinflammatory cytokines and chemokines (Lokensgard et al. 2001; Aravalli et al. 2005). In contrast to microglia, we found that astrocytes and neurons supported productive virus replication, but these brain cell types did not produce immune mediators in response to HSV-1 infection. In a subsequent study, we went on to demonstrate that HSV-1-induced immune mediator production by microglial cells was mediated through TLR2 signaling (Aravalli et al. 2005). Interestingly, when the human neuronal cell line NT2-N was infected with HSV-1 or rabies virus (RABV), differential expression of genes involved in immunity was observed (Prehaud et al. 2005). While HSV-1 induced the expression of mRNAs for IL-6 and IRF-1, RABV triggered the transcription of IL-6, TNF-α, IL-1α, CCL5, CXCL10, and IFN-β responsive genes. It is known that the activation of TLR3 leads to the production of IFN-β suggesting a response to the dsRNA intermediate produced during RABV replication. Thus, TLR3 signaling is involved in the differential generation of immune mediators by NT2-N cells in response to two different neurotropic viruses (Prehaud et al. 2005).
West Nile Virus (WNV) causes a febrile illness that can lead to lethal encephalitis or development of flaccid paralysis and long-term cognitive dysfunction, and studies in our laboratory have demonstrated that this virus triggers the release of cytokines and chemokines from human microglia (Cheeran et al. 2005). WNV has been shown to activate TLR3 in peripheral lymphoid tissue, leading to the production of TNF-α and permeability changes in the blood–brain barrier (BBB) (Wang et al. 2004). It has been suggested that TNF-α permeabilizes the BBB, providing WNV entry into the CNS. During its replication cycle, WNV forms a dsRNA intermediate that activates TLR3 signaling. Although WNV-infected TLR3−/− mice have higher viral loads, they were found to be deficient in the production of IFN-β, TNF-α, and IL-6. When compared with wild-type mice, TLR3−/− mice also had significantly lower mortality and reduced microglial activation after infection (Wang et al. 2004). As an extension to this study, Town et al. (2006) found that murine microglial cells respond to dsRNA by activating TLR3 signaling from endosomes and function as key sensors for viruses that produce dsRNA.
Bacteria are the most serious cause of acute meningitis in children and adults. In neonates, the common pathogens are Escherichia coli and GBS, whereas in infants and older children, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, and Streptococcus pneumoniae are more common. Pyrogenic infections of the brain parenchyma (brain abscess) are caused by a variety of Gram-positive and Gram-negative bacteria, and these serious infections (mortality about 20% with treatment) are associated with seizures, loss of mental acuity, and lesion site-dependent focal neurological defects (Mathiesen and Johnson 1997; Townsend and Scheld 1998). Furthermore, in addition to causing meningitis, localized Mycobacterium tuberculosis can cause infection or tuberculoma in the brain parenchyma, and localized Toxoplasma gondii or fungal brain abscesses occur most commonly in immunodeficient or immunosuppressed patients, especially those with AIDS. Hypothetically, interactions of these bacterial parasitic and fungal pathogens or their cell wall products with TLRs on brain cells trigger neuropathological effects that culminate in brain damage or death.
All of these neurotropic pathogens have been shown to signal through one or more TLR (Table 1). In the brain, microglial cells were shown to respond to S. aureus PGN through TLR2 by producing TNF-α, IL-1β, and IL-8 (Esen and Kielian 2005; Kielian et al. 2005, 2006). TLR2 was also shown to participate in the generation of immune responses to S. pneumoniae in an experimental meningitis model (Koedel et al. 2003, 2004). Similarly, LPS present in the cell wall of Gram-negative bacteria such as E. coli, H. influenzae, and N. meningitidis, triggers signaling through TLR4 that could elicit the inflammatory response in acute meningitis. T. gondii loads in the brain tissues of TLR2−/− and MyD88−/− mice were found to be significantly higher than in wild-type or TLR4−/− mice, suggesting that TLR2 is important in protection against T. gondii infection (Mun et al. 2003).
GBS are a major cause of neonatal meningitis and sepsis (Koedel et al. 2003, 2004). Heat-inactivated GBS, as well as a secreted protein factor GBS-F, has been found to induce apoptosis via the activation of TLR2 on murine microglial cells. In this case, NO-dependent cell death was found to take place in microglia derived from wild-type mice, but not in TLR2−/− or MyD88−/− animals, suggesting that bacterial infection could cause oxidative stress in murine microglia (Lehnardt et al. 2006). In the case of N. meningitidis, it has been reported that the major proinflammatory molecule LPS, both cell-wall-bound and secreted forms, can induce signaling through TLR4 and TLR2 (Mogensen et al. 2006a). Interestingly, however, it was also demonstrated that the outer membrane of N. meningitidis that is devoid of LPS was still able to induce the production of immune mediators, suggesting the involvement of an unknown PRR in the recognition of this bacterium (Humphries et al. 2005). It was shown earlier that different strains of N. meningitidis are recognized either by TLR2 or TLR4 (Mogensen 2006b) and that the signaling could involve multiple TLRs both in MyD88-dependent and MyD88-independent pathways (Humphries et al. 2005; Mogensen et al. 2006a, 2006b). The PRR that recognizes H. influenzae has not yet been identified in brain cells. In human monocytes and mouse macrophages TLR2 recognizes this bacterium (Galdiero et al. 2004), whereas TLR4 and MyD88 are critical in the lung (Wieland et al. 2005), and TLR2 and TLR4 are important in human peripheral blood mononuclear cells (Mogensen et al. 2006a).
Plasmodium falciparum is the cause of cerebral malaria (CM), which is responsible for an estimated 1–2 million deaths per year worldwide. Plasmodium berghei ANKA (PbA), a strain used in murine models of CM, was shown to elicit less severe disease in mice lacking MyD88 than wild-type and TRIF−/− mice (Coban et al. 2007). Further studies with mice demonstrated that TLR2 and TLR9 were involved in CM pathogenesis. Thus, both TLR2 and TLR9 recognize P. berghei ANKA and immune responses to counter infection are signaled through these receptors.
Mycobacterium tuberculosis is an important cause of meningitis and brain parenchymal infection, especially in developing countries and in immunocompromised patients. Microglia are the principal brain cells infected by this bacterium and, therefore, play an important role in the neuropathogenesis of CNS disease. When human microglia are infected with M. tuberculosis strain H37Rv, they respond by inducing a robust expression of cytokines and chemokines (Rock et al. 2005). It has been shown in a variety of mammalian cell types that TLR2 recognizes mycobacteria through their glycopeptidolipid structures (Quesniaux et al. 2004; Sweet and Schorey 2006). Therefore, it is likely that microglia respond to mycobacteria through TLR2 to induce proinflammatory immune mediators.
It has been well established that the movement of leukocytes into the CNS is a tightly regulated mechanism (Springer 1994). Recent evidence suggests that memory lymphocytes survey immune-privileged sites such as the brain to detect the presence of pathogens (reviewed by Engelhardt 2006). Valuable information on leukocyte entry into the CNS has been obtained from numerous studies on EAE (Engelhardt 2006) and the involvement of adhesion molecules in the homing of lymphocytes in the CNS was studied with several disease models such as Semliki Forest virus (Smith et al. 2000), Borna disease virus (Rubin et al. 1998), and meningoencephalitis induced by Trypanosoma cruzi (Roffe et al. 2003). The common theme emerging from these studies is that under inflammatory conditions circulating lymphocyes and monocytes readily gain access to the CNS and home to the sites of infection. A number of studies have shown that both B and T cells (cytotoxic, regulatory, and conventional CD4+ helper) as well as monocytes express TLRs (reviewed by Sutmuller et al. 2006 and Medvedev et al. 2006). It is not known how signaling from TLRs in lymphocytes modulate immune pathways in the CNS.
Regulation of TLR signaling: balancing neuronal damage and regeneration
Modulation of TLR signaling is a potential therapeutic approach to block the production of proinflammatory molecules that cause several devastating CNS diseases. On the other hand, enhanced production of immune mediators, through activation of TLR signaling, may be beneficial in the context of brain defense against microbial infections as well as for vaccine development. To achieve both of these objectives, understanding the contribution of TLRs, their adaptors, and other accessory proteins is critical. A number of biotechnology companies are currently developing synthetic compounds to modulate TLR signaling. A number of these compounds, which function either as agonists or antagonists of TLRs, are currently undergoing different phases of clinical trials (Schmidt 2006; Travis 2007).
As we have discussed, microglial cells are the “pivotal” players in the innate regulation of inflammatory responses in the CNS, and TLRs appear to govern the generation of many, if not most, of the inflammatory mediators produced by activated microglia. However, microglia function as a “double-edged sword”. When activated, they rapidly migrate to sites of brain damage and clear debris to maintain the integrity of the CNS. These cells also produce neuroprotective growth factors and neurotrophins that may contribute to brain repair. But, when proinflammatory immune mediator production is uncontrolled, microglia may cause severe neuronal damage (Streit 2002; Streit et al. 2005). Thus, control of microglial cell activation is a key regulatory event in generating protective immune responses. TLR signaling could, therefore, be a valuable “control switch” by which microglial cell functions could be modulated.
Recent reports suggest the involvement of various cellular proteins in the activation and suppression of TLR signaling by different feedback mechanisms that may vary depending upon the nature of the stimulus. For instance, while TLR signaling can be activated by endogenous ligands such as hsp60, it is also inhibited by cellular proteins such as the single immunoglobulin IL-1R-related molecule (SIGIRR) (Ohashi et al. 2000; Wald et al. 2003). Similarly, the radioprotective protein 105 (RP105), a TLR4 homolog recently identified in B cells, interacts with MD-1 and suppresses TLR4/MD-2 signaling by preventing the binding of microbial ligands to the TLR4/MD-2 complex (Divanovic et al. 2005a, b). Mice lacking either RP105 or MD-1 are hyporesponsive to LPS (Ogata et al. 2000; Nagai et al. 2002). Like TLR4, RP105 is present in a wide range of antigen-presenting cells and functions as a negative regulator of TLR4. This protein lacks the signaling domain and resembles the structure of a TLR inhibitor (Divanovic et al. 2005a). In vivo, RP105 has been shown to affect LPS responses in macrophages and dendritic cells.
Alternative splicing of mRNA to generate different protein isoforms with distinct functions is a mechanism for regulating signal transduction pathways. It has been well established that protein isoforms produced in this manner often possess both agonistic and antagonistic functions. In the past 3–4 years, isoforms for several proteins involved in TLR pathways have been identified. For example, the interleukin-1 (IL-1) receptor-associated kinase family member IRAK2 is alternatively spliced into four isoforms, of which two are inhibitory proteins, whereas the others have been shown to potentiate LPS-induced NF-κB activation (Hardy and O’Neill 2004). In studies with IRAK1−/− mice, it was demonstrated recently that IRAK-1 plays an important role in the host response to staphylococcal sepsis (Verdrengh et al. 2004). The IRAK1 splice variant IRAK1c, which lacks exon 11 of the IRAK gene, is predominantly expressed in the brain (Rao et al. 2005). Although IRAK1c interacts strongly with TLR signaling proteins MyD88, Tollip, IRAK2, and TRAF6, it does not activate downstream events as it does not possess kinase activity. The lack of NF-κB activation caused by overexpression of IRAK1c was shown to cause the blockade of IL-1β-, LPS- and CpG-induced production of TNF-α in multiple cell types. Thus, IRAK1c was thought to function as a dominant negative protein and regulates TLR-induced inflammation (Rao et al. 2005). This protein isoform was found to be predominantly expressed in human brain tissues isolated from two donors of 32 and 42 years of age and these tissues did not express full-length IRAK1. In contrast, both isoforms were present in equal amounts in brain tissues collected from two donors 62 and 71 years of age, and IRAK1 was most prominent in brain tissue from another 72-year-old donor (Su J et al. 2007). Based on these observations, it has been proposed that IRAKc may keep the brain in a resting noninflammatory state and that differential splicing of IRAK1 may correlate with age. These finding have obvious implications in diseases associated with aging such as Alzheimer’s and Parkinson’s diseases.
In addition to producing proinflammatory cytokines and chemokines in response to infections, activated microglial cells also generate various free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), and these free radicals can be neurotoxic (Halliwell 2006; Block et al. 2007). Emerging evidence suggests a link between TLR signaling and oxidative stress, as demonstrated recently in mammalian cells where the activation of NF-κB through TLR signaling was shown to induce the synthesis of ROS and RNS (Frantz et al. 2001; Ryan et al. 2004; Shishido et al. 2006; Nakahira et al. 2006). Recent studies also demonstrated that the activation of TLR2, TLR4, and TLR9 with multiple ligands resulted in the production of NO by microglial cells (Dalpke et al. 2002; Ebert et al. 2005), iNOS gene expression, and NO synthesis in monocytes (He et al. 2006). Furthermore, LPS-induced TLR4 signaling led to ROS-stimulated release of thioredoxin and MAP kinase activation, similar to H2O2 pathway, in dendritic cells and splenocytes (Matsuzawa et al. 2005).
In mouse macrophages, the potent antioxidant molecule heme oxygenase (HO)-1, induced by heme-containing moieties during oxidative stress generates the production of the antioxidant carbon monoxide (CO) (Nakahira et al. 2006). CO has been shown to inhibit TLR2, 4, 5, and 9 pathways. In HO-1−/− macrophages, increased production of free radicals as well as TNF-α were detected, and systemic inflammation was enhanced (Nakahira et al. 2006). It remains to be seen whether a similar mechanism of TLR inhibition by CO operates in microglial cells during oxidative stress induced by neurotropic pathogens.
In addition to modulating the production of deleterious proinflammatory immune mediators, the balance toward neuroprotection, neuronal damage and repair, can hypothetically be favored by preventing cell death (apoptosis). Accumulating evidence suggests that TLR signaling may lead to apoptosis. For example, activation of TLR4 by LPS can induce apoptosis of epithelial and endothelial cells (Bannerman and Goldblum 2003; Neff et al. 2006), and TLR ligands such as LPS, Pam, and poly(I:C) can induce apoptosis in macrophages, fibroblasts, and dendritic cells (Hsu et al. 2004; De Trez et al. 2005; Fischer et al. 2005). In a human monocytic cell line, bacterial lipoproteins that activate TLR2 were found to trigger apoptosis (Aliprantis et al. 1999), and Jung et al. (2005) showed that stimulation of murine microglial cells with LPS induced cell death in vitro, indicating that TLR4 was involved in causing apoptosis. Interestingly, the TLR2 ligands PGN, PTA, and Pam3Cys-Ser-Lys4 were unable to induce apoptosis in these cells (Jung et al. 2005). In contrast to findings with synthetic ligands, we found that murine microglial cells undergo apoptosis after infection with HSV and that this programmed cell death is dependent upon TLR2 activation. Other investigators have shown that in the cortex and hippocampus of LPS-treated rats, induction of apoptosis correlated with ROS production, indicating a potential link between TLR signaling, oxidative stress, and apoptosis (Aliprantis et al. 2001; Nolan et al. 2003). Induction of apoptosis by LPS might also depend on NO production as shown for vascular smooth muscle cells (Smith et al. 1998). Thus, it appears that during LPS-induced apoptosis, ROS and/or RNS play critical roles downstream of TLR4 signaling.
Caveats and conclusions
The past decade has witnessed a surge in research activity in the field of innate immunity. This increased interest has resulted in the identification of key molecules, receptors, and signaling pathways that initiate immune responses and eventually lead to the establishment of adaptive immunity. Studies investigating TLRs and their signaling mechanisms have also provided new insights into their involvement in defense against and damage caused by CNS infections. Despite this phenomenal progress, a number of challenges lie ahead in fully understanding the interaction of a given microbial ligand with its cognate TLR. For example, current evidence regarding the binding or interaction of any ligand with TLRs is largely circumstantial. Further studies, both biochemical as well as structural, are needed to demonstrate the nature of these interactions and to elucidate how a given TLR can recognize several different ligands, either alone or as heterodimers in association with other TLRs and coreceptors.
Many studies demonstrating the role of TLRs in CNS pathogenesis have relied on in vitro techniques that necessitate isolation of highly purified brain cell cultures. Such studies can generate misleading information related to the artificial nature of in vitro studies, or the lack of purity of the brain cell types under investigation or by contamination by microbial products such as LPS. Seminal studies using TLR knockout animals have resulted in mapping intracellular signaling pathways. However, because of the potential redundancy in TLR activation, double knockout animals would be valuable in identifying key molecules and mechanisms. A recent report on HSV infection of dendritic cells has demonstrated sequential activation of TLR2 and TLR9 in response to the virus (Sato et al. 2006). Future studies using animals with multiple TLR knockouts will be useful in determining the mechanisms responsible for regulating TLR-driven innate neuroimmune responses.
Therapies designed to alleviate the damaging effects of TLR-induced production of inflammatory mediators (cytokines, chemokines, and free radicals) in the CNS will be aimed at modulation of TLR signaling. Although a large number of antibodies and synthetic molecules that interact with TLRs are commercially available, specific molecules that anatagonize or inhibit TLR signaling are still not available. Most of these antibodies, including those marketed as TLR2 antagonists, function as agonists of TLR signaling as well. Therefore, one of the key challenges that lie ahead is the development of specific inhibitors of TLRs and the identification of intracellular signaling pathways that are affected by these inhibitors.
References
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Aliprantis AO, Weiss DS, Zychlinsky A (2001) Toll-like receptor-2 transduces signals for NF-kappa B activation, apoptosis and reactive oxygen species production. J Endotoxin Res 7:287–291
Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A (1999) Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor 2. Science 285:736–739
Aloisi F (2001) Immune function of microglia. Glia 36:165–179
Aravalli RN, Hu S, Rowen TN, Palmquist J, Lokensgard JR (2005) Cutting Edge: TLR2-mediated production of proinflammatory cytokines and chemokines by microglial cells in response to herpes simplex virus. J Immunol 175:4189–4193
Aravalli RN, Hu S, Rowen TN, Gekker G, Lokensgard JR (2006) Differential apoptotic signaling in primary glial cells infected with herpes simplex virus 1. J Neurovirol 12:501–510
Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25:187–191
Aschner M, Allen JW, Kimelberg HK, LoPachin RM, Streit WJ (1999) Glial cells in neurotoxicity development. Annu Rev Pharmacol Toxicol 39:151–173
Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034
Banati RB, Gehrmann J, Schubert P, Kreutzberg GW (1993) Cytotoxicity of microglia. Glia 7:111–118
Bandholtz L, Guo Y, Palmberg C, Mattsson K, Ohlsson B, High A, Shabanowitz J, Hunt DF, Jornvall H, Wigzell H, Agerberth B, Gudmundsson GH (2003) Hsp90 binds CpG oligonucleotides directly: implications for hsp90 as a missing link in CpG signaling and recognition. Cell Mol Life Sci 60:422–429
Bannerman DD, Goldblum SE (2003) Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol 284:L899–L914
Becher B, Fedorowicz V, Antel JP (1996) Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res 45:375–381
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW (2002) Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science 298:1025–1029
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Bowman CC, Rasley A, Tranguch SL, Marriott I (2003) Cultured astrocytes express toll-like receptors for bacterial products. Glia 43:281–291
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021
Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM (2006) Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53:688–695
Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M (2002) Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol 168:1435–1440
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49:360–374
Chakravarty S, Herkenham M (2005) Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25:1788–1796
Chan G, Guilbert LJ (2006) Ultraviolet-inactivated human cytomegalovirus induces placental syncytiotrophoblast apoptosis in a Toll-like receptor-2 and tumour necrosis factor-a dependent manner. J Pathol 210:111–120
Chao CC, Molitor TW, Hu S (1993) Neuroprotective role of IL-4 against activated microglia. J Immunol 151:1473–1481
Cheeran MC, Hu S, Sheng WS, Rashid A, Peterson PK, Lokensgard JR (2005) Differential responses of human brain cells to West Nile virus infection. J Neurovirol 11:512–524
Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM (1996) Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun 64:1906–1912
Coban C, Ishii KJ, Uematsu S, Arisue N, Sato S, Yamamoto M, Kawai T, Takeuchi O, Hisaeda H, Horii T, Akira S (2007) Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol 19:67–79
Cooke GS, Hill AV (2001) Genetics of susceptibility to human infectious disease. Nat Rev Genet 2:967–977
Creagh EM, O’Neill LAJ (2006) TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 27:352–357
Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K (2002) Immunostimulatory CpG-DNA activates murine microglia. J Immunol 168:4854–4863
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136
De Trez C, Pajak B, Brait M, Glaichenhaus N, Urbain J, Moser M, Lauvau G, Muraille E (2005) TLR4 and Toll-IL-1 receptor domain-containing adapter-inducing IFNbeta, but not MyD88, regulate Escherichia coli-induced dendritic cell maturation and apoptosis in vivo. J Immunol 175:839–846
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531
Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL (2005a) Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol 6:571–578
Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL (2005b) Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res 11:363–368
Dobrovolskaia MA, Vogel SN (2002) Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect 4:903–914
Draper DW, Bethea HN, He YW (2006) Toll-like receptor 2-dependent and -independent activation of macrophages by group B streptococci. Immunol Lett 102:202–214
Dziarski R, Gupta D (2000) Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes. J Endotoxin Res 6:401–405
Dziarski R, Jin YP, Gupta D (1996) Differential activation of extracellular signal-regulated kinase (ERK) 1, ERK2, p38, and c-Jun NH2-terminal kinase mitogen-activated protein kinases by bacterial peptidoglycan. J Infect Dis 174:777–785
Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D (2001) MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol 166:1938–1944
Ebert S, Gerber J, Bader S, Muhlhauser F, Brechtel K, Mitchell TJ, Nau R (2005) Dosedependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J Neuroimmunol 159:87–96
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 100:13632–13637
Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the blood–brain barrier. J Neural Transm 113:477–485
Esen N, Kielian T (2005) Recognition of Staphylococcus aureus-derived peptidoglycan (PGN) but not intact bacteria is mediated by CD14 in microglia. J Neuroimmunol 170:93–104
Esen N, Kielian T (2006) Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol 176:6802–6811
Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E (2005) Preferential expression and function of Toll-like receptor 3 in human astrocytes. J Neuroimmunol 159:12–19
Fearon DT (1997) Seeking wisdom in innate immunity. Nature 388:323–324
Fischer SF, Rehm M, Bauer A, Hofling F, Kirschnek S, Rutz M, Bauer S, Wagner H, Hacker G (2005) Toll-like receptor 9 signaling can sensitize fibroblasts for apoptosis. Immunol Lett 97:115–122
Frantz S, Kelly RA, Bourcier T (2001) Role of TLR-2 in the activation of nuclear factor κB by oxidative stress in cardiac myocytes. J Biol Chem 276:5197–5203
Galdiero M, Galdiero M, Finamore E, Rossano F, Gambuzza M, Catania MR, Teti G, Midiri A, Mancuso G (2004) Haemophilus influenzae porin induces Toll-like receptor 2-mediated cytokine production in human monocytes and mouse macrophages. Infect Immun 72:1204–1209
Frodsham AJ, Hill AV (2004) Genetics of infectious diseases. Human Mol Genet:R187–R194 (Spec No. 182)
Gao JJ, Xue Q, Zuvanich EG, Haghi KR, Morrison, DC (2001) Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect Immun 69:751–757
Goldstein DB, Cavalleri GL (2005) Genomics: understanding human diversity. Nature 437:1241–1242
Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M (2002) Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168:5989–5992
Gupta D, Kirkland TN, Viriyakosol S, Dziarski R (1996) CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem 271:23310–23316
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Hardy MP, O’Neill LA (2004) The murine IRAK2 gene encodes four alternatively spliced isoforms, two of which are inhibitory. J Biol Chem 279:27699–27708
Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE (2005) Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol 174:2942–2950
Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of Drosophila, required for dorsal–ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269–279
He H, Genovese KJ, Nisbet DJ, Kogut MH (2006) Profile of Toll-like receptor expressions and induction of nitric oxide synthesis by Toll-like receptor agonists in chicken monocytes. Mol Immunol 43:783–789
Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT (2001) Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol 167:7069–7076
Hoffmann JA, Kafatos FC, Janeway CA, Jr, Ezekowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science 284:1313–1318
Honig LS, Rosenberg RN (2000) Apoptosis and neurologic disease. Am J Med 108:317–330
Hosoi T, Suzuki S, Nomura J, Ono A, Okuma Y, Akira S, Nomura Y (2004) Bacterial DNA induced iNOS expression through MyD88-p38 MAP kinase in mouse primary cultured glial cells. Brain Res Mol Brain Res 124:159–164
Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M (2004) The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428:341–345
Humphries HE, Triantafilou M, Makepeace BL, Heckels JE, Triantafilou K, Christodoulides M (2005) Activation of human meningeal cells is modulated by lipopolysaccharide (LPS) and non-LPS components of Neisseria meningitidis and is independent of Toll-like receptor 4 and TLR2 signaling. Cell Microbiol 7:415–430
Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175:4320–4330
Jin FY, Nathan C, Radzioch D, Ding A (1997) Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell 88:417–426
Johnson GB, Brunn GJ, Kodaira Y, Platt JL (2002) Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168:5233–5239
Johnson GB, Brunn GJ, Platt JL (2003) Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol 23:15–44
Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, Suk K (2005) TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol 174:6467–6476
Kang TJ, Chae GT (2001) Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol Med Microbiol 31:53–58
Kariko K, Ni H, Capodici J, Lamphier M, Weissman D (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279:12542–12550
Kawai T, Akira S (2006) TLR signaling. Cell Death Differ 13:816–825
Kerfoot SM, Long EM, Hickey MJ, Andonegui G, Lapointe BM, Zanardo RC, Bonder C, James WG, Robbins SM, Kubes P (2004) TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J Immunol 173:7070–7077
Kiechl S, Lorenz E, Reindl M, Wiedermann CJ, Oberhollenzer F, Bonora E, Willeit J, Schwartz DA (2002) Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 347:185–192
Kielian T, Esen N, Bearden ED (2005) Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia 49:567–576
Kielian T, Haney A, Mayes PM, Garg S, Esen N (2006) Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect Immun 73:7428–7435
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81:302–313
Kitamura Y, Spleiss O, Li H, Taniguchi T, Kimura H, Nomura Y, Gebicke-Haerter PJ (2001) Lipopolysaccharide-induced switch between retinoid receptor (RXR) alpha and glucocorticoid attenuated response gene (GARG)-16 messenger RNAs in cultured rat microglia. J Neurosci Res 64:553–563
Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ (2003) Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol 170:438–444
Koedel U, Rupprecht T, Angele B, Heesemann J, Wagner H, Pfister HW, Kirschning CJ (2004) MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain Res 127:1437–1445
Laflamme N, Soucy G, Rivest S (2001) Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem 79:648–657
Laflamme N, Echchannaoui H, Landmann R, Rivest S (2003) Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur J Immunol 33:1127–1138
Lee SJ, Lee S (2002) Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy 1:181–191
Lee S, Hong J, Choi SY, Oh SB, Park K, Kim JS, Karin M, Lee SJ (2004) CpG oligodeoxynucleotides induce expression of proinflammatory cytokines and chemokines in astrocytes: the role of c-Jun N-terminal kinase in CpG ODN-mediated NF-kappaB activation. J Neuroimmunol 153:50–63
Lee H, Jo EK, Choi SY, Oh SB, Park K, Soo Kim J, Lee SJ (2006) Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem Biophys Res Commun 350:742–747
Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22:2478–2486
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 100:8514–8519
Lehnardt S, Henneke P, Lien E, Kasper DL, Volpe JJ, Bechmann I, Nitsch R, Weber JR, Golenbock DT, Vartanian T (2006) A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol 177:583–592
Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA (1996) The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86:973–983
Lokensgard JR, Hu S, Sheng W, van Oijen M, Cox D, Cheeran MC, Peterson PK (2001) Robust expression of TNF-alpha, IL-1beta, RANTES, and IP-10 by human microglial cells during nonproductive infection with herpes simplex virus. J Neurovirol 7:208–219
Lorenz E, Patel DD, Hartung T, Schwartz DA (2002) Toll-like receptor 4 (TLR4)-deficient murine macrophage cell line as an in vitro assay system to show TLR4-independent signaling of Bacteroides fragilis lipopolysaccharide. Infect Immun 70:4892–4896
Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lu J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T (2006) Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol 175:209–215
Majde JA (1993) Microbial cell wall contaminants in peptides: a potential source of physiological artifacts. Peptides 14:629–632
Malhotra D, Relhan V, Reddy BS, Bamezai R (2005) TLR2 Arg677Trp polymorphism in leprosy: revisited. Hum Genet 116:413–415
Manukyan M, Triantafilou K, Triantafilou M, Mackie A, Nilsen N, Espevik T, Wiesmuller KH, Ulmer AJ, Heine H (2005) Binding of lipopeptide to CD14 induces physical proximity of CD14, TLR2 and TLR1. Eur J Immunol 35:911–921
Mathiesen GE, Johnson JP (1997) Brain abscess. Clin Infect Dis 25:763–779
Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H (2005) ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6:587–592
McKimmie CS, Fazakerley JK (2005) In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol 169:116–125
Medvedev AE, Flo T, Ingalls RR, Golenbock DT, Teti G, Vogel SN, Espevik T (1998) Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol 160:4535–4542
Medvedev AE, Sabroe I, Hasday JD, Vogel SN (2006) Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res 12:133–150
Medzhitov R, Preston-Hurlburt P, Janeway CA, Jr (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394–397
Miggin SM, O’Neill LA (2006) New insights into the regulation of TLR signaling. J Leukoc Biol 80:220–226
Mockenhaupt FP, Cramer JP, Hamann L, Stegemann MS, Eckert J, Oh NR, Otchwemah RN, Dietz E, Ehrhardt S, Schroder NW, Bienzle U, Schumann RR (2006) Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci U S A 103:177–182
Mogensen TH, Paludan SR, Kilian M, Ostergaard L (2006a) Live Streptococcus penumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol 80:267–277
Mogensen TH, Paludan SR, Kilian M, Ostergaard L (2006b) Two neisseria meningitidis strains with different ability to stimulate Toll-like receptor 4 through the MyD88-independent pathway. Scan J Immunol 64:646–654
Mun HS, Aosai F, Narose K, Chen M, Piao LX, Takeuchi O, Akira S, Ishikura H, Yano A (2003) TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol 15:1081–1087
Nagai Y, Shimazu R, Ogata H, Akashi S, Sudo K, Yamasaki H, Hayashi S-I, Iwakura Y, Kimoto M, Miyake K (2002) Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide. Blood 99:1699–1705
Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Wang X, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM (2006) Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 203:2377–2389
Neff SB, Z’graggen BR, Neff TA, Jamnicki-Abegg M, Suter D, Schimmer RC, Booy C, Joch H, Pasch T, Ward PA, Beck-Schimmer B (2006) Inflammatory response of tracheobronchial epithelial cells to endotoxin. Am J Physiol Lung Cell Mol Physiol 290:L86–L96
Nolan Y, Vereker E, Lynch AM, Lynch MA (2003) Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Exp Neurol 184:794–804
Ogata H, Su I-H, Miyake K, Nagai Y, Akashi S, Mecklenbrauker I, Rajewsky K, Kimoto M, Tarakhovsky A (2000) The Toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med 192:23–29
Ogus AC, Yoldas B, Ozdemir T, Uguz A, Olcen S, Keser I, Coskun M, Cilli A, Yegin O (2004) The Arg753GLn polymorphism of the human toll-like receptor 2 gene in tuberculosis disease. Eur Respir J 23:219–223
Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558–561
Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y (2006) Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 45:8657–8664
Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF 3rd (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276:10229–10233
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173:3916–3924
Pahlman LI, Morgelin M, Eckert J, Johansson L, Russell W, Riesbeck K, Soehnlein O, Lindbom L, Norrby-Teglund A, Schumann RR, Bjorck L, Herwald H (2006) Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol 177:1221–1228
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377
Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ (2006) TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia 53:248–256
Paul WE (1998) Fundamental immunology, 4th edn. Lippincott-Raven Publishers, Philadelphia
Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF (2005) Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309:1380–1384
Prehaud C, Megret F, Lafage M, Lafon M (2005) Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol 79:12893–12904
Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Bruck W, Becher B (2006) Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest 116:456–464
Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML (2005) Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 52:78–84
Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, Soler MN, Le Bert M, Schnyder B, Ryffel B (2004) Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect 6:946–959
Rao N, Nguyen S, Ngo K, Fung-Leung WP (2005) A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol Cell Biol 25:6521–6532
Rasley A, Anguita J, Marriott I (2002) Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J Neuroimmunol 130:22–31
Read RC, Pullin J, Gregory S, Borrow R, Kaczmarski EB, di Giovine FS, Dower SK, Cannings C, Wilson AG (2001) A functional polymorphism of toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J Infect Dis 184:640–642
Reed RC, Berwin B, Baker JP, Nicchitta CV (2003) GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-kappa B activation and nitric oxide production. J Biol Chem 278:31853–31860
Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A (2005) The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A 102:9577–9582
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK (2004) Role of microglia in central nervous system infections. Clin Microbiol Rev 17:942–964
Rock RB, Hu S, Gekker G, Sheng WS, May B, Kapur V, Peterson PK (2005) Mycobacterium tuberculosis-induced cytokine and chemokine expression by human microglia and astrocytes: effects of dexamethasone. J Infect Dis 192:2054–2058
Roffe E, Silva AA, Marino AP, dos Santos PV, Lannes-Vieira J (2003) Essential role of VLA-4/VCAM-1 pathway in the establishment of CD8+ T-cell mediated Trypanosoma cruzi-elicited meningoencephalitis. J Neuroimmunol 142:17–30
Rubin SA, Yednock TA, Carbone KM (1998) In vivo treatment with anti-alpha4 integrin suppresses clinical and pathological evidence of Borna disease virus infection. J Neuroimmunol 84:158–163
Ryan KA, Smith MF Jr, Sanders MK, Ernst PB (2004) Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-κB and interleukin-8 expression. Infect Immun 72:2123–2130
Sato A, Linehan MM, Iwasaki A (2006) Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A 103:17343–17348
Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177:1272–1281
Schmidt C (2006) Toll-like receptor therapies compete to reduce side effects. Nat Biotechnol 24:230–231
Schork NJ, Fallin D, Lanchbury JS (2000) Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet 58:250–264
Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR (2003) Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 278:15587–15594
Schroder NW, Diterich I, Zinke A, Eckert J, Draing C, von Baehr V, Hassler D, Priem S, Hahn K, Michelsen KS, Hartung T, Burmester GR, Gobel UB, Hermann C, Schumann RR (2005) Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol 175:2534–2540
Scumpia PO, Kelly KM, Reeves WH, Stevens BR (2005) Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia 52:153–162
Segal S, Hill AV (2003) Genetic susceptibility to infectious disease. Trends Microbiol 11:445–448
Shishido T, Nozaki N, Takahashi H, Arimoto T, Niizeki T, Koyama Y, Abe J, Takeishi Y, Kubota I (2006) Central role of endogenous Toll-like receptor 2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem Biophys Res Commun 345:1446–1453
Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167:2887–2894
Smirnova I, Mann N, Dols A, Derkx HH, Hibberd ML, Levin M, Beutler B (2003) Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc Natl Acad Sci U S A 100:6075–6080
Smith JD, McLean SD, Nakayama DK (1998) Nitric oxide causes apoptosis in pulmonary vascular smooth muscle cells. J Surg Res 79:121–127
Smith JP, Morris-Downes M, Brennan FR, Wallace GJ, Amor S (2000) A role for alpha4-integrin in the pathology following Semliki Forest virus infection. J Neuroimmunol 106:60–68
So EY, Kang MH, Kim BS (2006) Induction of chemokine and cytokine genes in astrocytes following infection with Theiler’s murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858–867
Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301–314
Stoll G, Jander S, Myers RR (2002) Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher Nerv Syst 7:13–27
Streit WJ (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133–139
Streit WJ, Mrak RE, Griffin WST (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflamation 1:14
Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL (2005) Role of microglia in the central nervous system’s immune response. Neurol Res 27:685–691
Su J, Richter K, Zhang C, Gu Q, Li L. (2007) Differential regulation of interleukin-1 receptor associated kinase 1 (IRAK1) splice variants. Mol Immunol 44:900–905
Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ (2006) Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol 27:387–393
Sweet L, Schorey JS (2006) Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J Leukoc Biol 80:415–423
Takeshita S, Takeshita F, Haddad DE, Janabi N, Klinman DM (2001) Activation of microglia and astrocytes by CpG oligodeoxynucleotides. NeuroReport 12:3029–3032
Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC (2002) Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195:99–111
Town T, Jeng D, Alexopoulou L, Tan J, Flavell RA (2006) Microglia recognize double-stranded RNA via TLR3. J Immunol 176:3804–3812
Townsend GC, Scheld WM (1998) Infections of the central nervous system. Adv Intern Med 43:403–447
Travis K (2007) Deciphering immunology’s dirty little secret. The Scientist 21:46–49
Tsan MF, Gao B (2004) Endogenous ligands of Toll-like receptors. J Leukoc Biol 76:514–519
Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H (2002a) HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277:15107–15112
Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H (2002b) The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem 277:20847–20853
Verdrengh M, Thomas JA, Hultgren O (2004) IL-1 receptor-associated kinase 1 mediates protection against Staphylococcus aureus infection. Microbes Infect 6:1268–1272
Visser L, de Heer HJ, Boven LA, van Riel D, van Meurs M, Melief MJ, Zahringer U, van Strijp J, Lambrecht BN, Nieuwenhuis EE, Laman JD (2005) Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol 174:808–816
Wadachi R, Hargreaves KM (2006) Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res 85:49–53
Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol 4:920–927
Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling TE, Langenbach R, Taniura S, Hong JS (2005) Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem 88:939–947
Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nature Med 10:1366–1373
Weber JR, Freyer D, Alexander C, Schroder NW, Reiss A, Kuster C, Pfeil D, Tuomanen EI, Schumann RR (2003) Recognition of pneumococcal peptidoglycan: an expanded, pivotal role for LPS binding protein. Immunity 19:269–2798
Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T (2005) The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol 175:6042–6049
Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629
Yim JJ, Lee HW, Lee HS, Kim YW, Han SK, Shim YS, Holland SM (2006a) The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun 7:150–155
Yim JJ, Adams AA, Kim JH, Holland SM (2006b) Evolution of an intronic microsatellite polymorphism in Toll-like receptor 2 among primates. Immunogenetics 58:740–745
Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H (2006) HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26:174–179
Zekki H, Feinstein DL, Rivest S (2002) The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol 12:308–319
Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S (2004) A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303:1481–1482
Zhang Z, Trautmann K, Schluesener HJ (2005a) Microglia activation in rat spinal cord by systemic injection of TLR3 and TLR7/8 agonists. J Neuroimmunol 164:154–160
Zhang Z, Guo K, Schluesener HJ (2005b) The immunostimulatory activity of CpG oligonucleotides on microglial N9 cells is affected by a polyguanosine motif. J Neuroimmunol 161:68–77
Zhou J, An H, Xu H, Liu S, Cao X (2005) Heat shock up-regulates expression of Toll-like receptor-2 and Toll-like receptor-4 in human monocytes via p38 kinase signal pathway. Immunology 114:522–530
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by United States Public Health Service Award MH066703.
Rights and permissions
About this article
Cite this article
Aravalli, R.N., Peterson, P.K. & Lokensgard, J.R. Toll-like Receptors in Defense and Damage of the Central Nervous System. J Neuroimmune Pharmacol 2, 297–312 (2007). https://doi.org/10.1007/s11481-007-9071-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-007-9071-5