Abstract
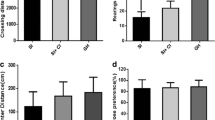
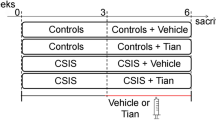
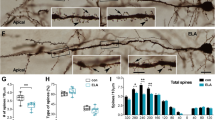
Our previous experiments demonstrated that social isolation (SI) caused AD-like tau hyperphosphorylation and spatial memory deficits in middle-aged rats. However, the underlying mechanisms of SI-induced spatial memory deficits remain elusive. Middle-aged rats (10 months) were group or isolation reared for 8 weeks. Following the initial 4-week period of rearing, citalopram (10 mg/kg i.p.) was administered for 28 days. Then, pathophysiological changes were assessed by performing behavioral, biochemical, and pathological analyses. We found that SI could cause cognitive dysfunction and decrease synaptic protein (synaptophysin or PSD93) expression in different brain regions associated with cognition, such as the prefrontal cortex, dorsal hippocampus, ventral hippocampus, amygdala, and caudal putamen, but not in the entorhinal cortex or posterior cingulate. Citalopram could significantly improve learning and memory and partially restore synaptophysin or PSD93 expression in the prefrontal cortex, hippocampus, and amygdala in SI rats. Moreover, SI decreased the number of dendritic spines in the prefrontal cortex, dorsal hippocampus, and ventral hippocampus, which could be reversed by citalopram. Furthermore, SI reduced the levels of BDNF, serine-473-phosphorylated Akt (active form), and serine-9-phosphorylated GSK-3β (inactive form) with no significant changes in the levels of total GSK-3β and Akt in the dorsal hippocampus, but not in the posterior cingulate. Our results suggest that decreased synaptic plasticity in cognition-associated regions might contribute to SI-induced cognitive deficits, and citalopram could ameliorate these deficits by promoting synaptic plasticity mainly in the prefrontal cortex, dorsal hippocampus, and ventral hippocampus. The BDNF/Akt/GSK-3β pathway plays an important role in regulating synaptic plasticity in SI rats.






Similar content being viewed by others
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362(4):329–344. doi:10.1056/NEJMra0909142
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6):1204–1222. doi:10.1016/j.cell.2012.02.040
Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG (2011) Social isolation. Ann N Y Acad Sci 1231:17–22. doi:10.1111/j.1749-6632.2011.06028.x
Fone KC, Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32(6):1087–1102. doi:10.1016/j.neubiorev.2008.03.003
Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P (2011) Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res 216(2):552–560. doi:10.1016/j.bbr.2010.08.038
McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J (2010) Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav Brain Res 208(1):23–29. doi:10.1016/j.bbr.2009.11.003
Jiang Z, Cowell RM, Nakazawa K (2013) Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front Behav Neurosci 7:116. doi:10.3389/fnbeh.2013.00116
Gilman SE, Ni MY, Dunn EC, Breslau J, McLaughlin KA, Smoller JW, Perlis RH (2015) Contributions of the social environment to first-onset and recurrent mania. Mol Psychiatry 20(3):329–336. doi:10.1038/mp.2014.36
Dong H, Csernansky JG (2009) Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis 18(2):459–469. doi:10.3233/JAD-2009-1152
Ren QG, Gong WG, Wang YJ, Zhou QD, Zhang ZJ (2015) Citalopram attenuates tau hyperphosphorylation and spatial memory deficit induced by social isolation rearing in middle-aged rats. J Mol Neurosci 56(1):145–153. doi:10.1007/s12031-014-0475-4
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368(9533):387–403. doi:10.1016/S0140-6736(06)69113-7
Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW Jr, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56(1):127–129
Honer WG (2003) Pathology of presynaptic proteins in Alzheimer’s disease: more than simple loss of terminals. Neurobiol Aging 24(8):1047–1062
Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ (2006) Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol 65(6):592–601
Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM (2000) Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97(2):253–266
Alfarez DN, Joels M, Krugers HJ (2003) Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci 17(9):1928–1934
Li XL, Yuan YG, Xu H, Wu D, Gong WG, Geng LY, Wu FF, Tang H et al (2015) Changed synaptic plasticity in neural circuits of depressive-like and escitalopram-treated rats. Int J Neuropsychopharmacol 18(10):pyv046. doi:10.1093/ijnp/pyv046
Tucker S, Ahl M, Bush A, Westaway D, Huang X, Rogers JT (2005) Pilot study of the reducing effect on amyloidosis in vivo by three FDA pre-approved drugs via the Alzheimer’s APP 5′ untranslated region. Curr Alzheimer Res 2(2):249–254
Ren QG, Wang YJ, Gong WG, Xu L, Zhang ZJ (2015) Escitalopram ameliorates tau hyperphosphorylation and spatial memory deficits induced by protein kinase A activation in Sprague Dawley rats. J Alzheimers Dis 47(1):61–71. doi:10.3233/JAD-143012
Ren QG, Wang YJ, Gong WG, Zhou QD, Xu L, Zhang ZJ (2015) Escitalopram ameliorates forskolin-induced tau hyperphosphorylation in HEK239/tau441 cells. J Mol Neurosci 56(2):500–508. doi:10.1007/s12031-015-0519-4
Hoe HS, Lee KJ, Carney RS, Lee J, Markova A, Lee JY, Howell BW, Hyman BT et al (2009) Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci 29(23):7459–7473. doi:10.1523/JNEUROSCI.4872-08.2009
Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K (2006) Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav 83(2):186–193. doi:10.1016/j.pbb.2006.01.004
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23. doi:10.1038/nrn3379
Steptoe A, Shankar A, Demakakos P, Wardle J (2013) Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A 110(15):5797–5801. doi:10.1073/pnas.1219686110
Weiss EM, Kohler CG, Vonbank J, Stadelmann E, Kemmler G, Hinterhuber H, Marksteiner J (2008) Impairment in emotion recognition abilities in patients with mild cognitive impairment, early and moderate Alzheimer disease compared with healthy comparison subjects. Am J Geriatr Psychiatry 16(12):974–980. doi:10.1097/JGP.0b013e318186bd53
Hsiao YH, Chen PS, Chen SH, Gean PW (2011) The involvement of Cdk5 activator p35 in social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit in the transgenic mice. Neuropsychopharmacology 36(9):1848–1858. doi:10.1038/npp.2011.69
Hsiao YH, Kuo JR, Chen SH, Gean PW (2012) Amelioration of social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit by N-acetylcysteine in a transgenic mouse model. Neurobiol Dis 45(3):1111–1120. doi:10.1016/j.nbd.2011.12.031
Huang H, Wang L, Cao M, Marshall C, Gao J, Xiao N, Hu G, Xiao M (2015) Isolation housing exacerbates Alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int J Neuropsychopharmacol 18(7):pyu116. doi:10.1093/ijnp/pyu116
Egashira N, Matsumoto Y, Mishima K, Iwasaki K, Fujioka M, Matsushita M, Shoyama Y, Nishimura R et al (2006) Low dose citalopram reverses memory impairment and electroconvulsive shock-induced immobilization. Pharmacol Biochem Behav 83(1):161–167. doi:10.1016/j.pbb.2006.01.006
Couto FS, Batalha VL, Valadas JS, Data-Franca J, Ribeiro JA, Lopes LV (2012) Escitalopram improves memory deficits induced by maternal separation in the rat. Eur J Pharmacol 695(1-3):71–75. doi:10.1016/j.ejphar.2012.08.020
Mowla A, Mosavinasab M, Haghshenas H, Borhani Haghighi A (2007) Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer’s dementia? A double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol 27(5):484–487. doi:10.1097/jcp.0b013e31814b98c1
Mowla A, Mosavinasab M, Pani A (2007) Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled, clinical trial. J Clin Psychopharmacol 27(1):67–70. doi:10.1097/JCP.0b013e31802e0002
Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Santamato A, Vendemiale G et al (2010) Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry 18(2):98–116. doi:10.1097/JGP.0b013e3181b0fa13
Miklowitz DJ (2011) Functional impairment, stress, and psychosocial intervention in bipolar disorder. Curr Psychiatry Rep 13(6):504–512. doi:10.1007/s11920-011-0227-x
Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y et al (2009) Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res 202(1):114–121. doi:10.1016/j.bbr.2009.03.028
Lukkes JL, Mokin MV, Scholl JL, Forster GL (2009) Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav 55(1):248–256. doi:10.1016/j.yhbeh.2008.10.014
Ouchi H, Ono K, Murakami Y, Matsumoto K (2013) Social isolation induces deficit of latent learning performance in mice: a putative animal model of attention deficit/hyperactivity disorder. Behav Brain Res 238:146–153. doi:10.1016/j.bbr.2012.10.029
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27(5):457–464. doi:10.1002/ana.410270502
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 30(4):572–580. doi:10.1002/ana.410300410
Valtorta F, Tarelli FT, Campanati L, Villa A, Greengard P (1989) Synaptophysin and synapsin I as tools for the study of the exo-endocytotic cycle. Cell Biol Int Rep 13(12):1023–1038
Sheng M (1996) PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron 17(4):575–578
Kennedy MB (1997) The postsynaptic density at glutamatergic synapses. Trends Neurosci 20(6):264–268
Kornau HC, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269(5231):1737–1740
Kruger JM, Favaro PD, Liu M, Kitlinska A, Huang X, Raabe M, Akad DS, Liu Y et al (2013) Differential roles of postsynaptic density-93 isoforms in regulating synaptic transmission. J Neurosci 33(39):15504–15517. doi:10.1523/JNEUROSCI.0019-12.2013
Qiu A, Fennema-Notestine C, Dale AM, Miller MI, Alzheimer’s Disease Neuroimaging I (2009) Regional shape abnormalities in mild cognitive impairment and Alzheimer’s disease. Neuroimage 45(3):656–661
Zhao H, Li X, Wu W, Li Z, Qian L, Li S, Zhang B, Xu Y (2015) Atrophic patterns of the frontal-subcortical circuits in patients with mild cognitive impairment and Alzheimer’s disease. PLoS One 10(6):e0130017. doi:10.1371/journal.pone.0130017
Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, Fonov V, Jia J et al (2012) Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One 7(10):e47905. doi:10.1371/journal.pone.0047905
Xi Q, Zhao XH, Wang PJ, Guo QH, He Y (2013) Abnormal intrinsic brain activity in amnestic mild cognitive impairment revealed by amplitude of low-frequency fluctuation: a resting-state functional magnetic resonance imaging study. Chin Med J (Engl) 126(15):2912–2917
Leuner B, Shors TJ (2013) Stress, anxiety, and dendritic spines: what are the connections? Neuroscience 251:108–119. doi:10.1016/j.neuroscience.2012.04.021
Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE (2013) A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn 81(1):29–43. doi:10.1016/j.bandc.2012.10.005
Benson BE, Willis MW, Ketter TA, Speer A, Kimbrell TA, Herscovitch P, George MS, Post RM (2014) Differential abnormalities of functional connectivity of the amygdala and hippocampus in unipolar and bipolar affective disorders. J Affect Disord 168:243–253. doi:10.1016/j.jad.2014.05.045
Peng B, Wu L, Zhang L, Chen Y (2015) Volumetric changes in amygdala and entorhinal cortex and their relation to memory impairment in patients with medial temporal lobe epilepsy with visually normal MR imaging findings. Epilepsy Res 114:66–72. doi:10.1016/j.eplepsyres.2015.04.018
Zorrilla EP, Koob GF (2013) Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neurosci Biobehav Rev 37(9 Pt A):1932–1945. doi:10.1016/j.neubiorev.2012.11.019
Liu Q, Trotter J, Zhang J, Peters MM, Cheng H, Bao J, Han X, Weeber EJ et al (2010) Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J Neurosci 30(50):17068–17078. doi:10.1523/JNEUROSCI.4067-10.2010
Mavroudis IA, Manani MG, Petrides F, Petsoglou C, Njau SN, Costa VG, Baloyannis SJ (2014) Dendritic and spinal alterations of neurons from Edinger-Westphal nucleus in Alzheimer’s disease. Folia Neuropathol 52(2):197–204
Dorostkar MM, Zou C, Blazquez-Llorca L, Herms J (2015) Analyzing dendritic spine pathology in Alzheimer’s disease: problems and opportunities. Acta Neuropathol 130(1):1–19. doi:10.1007/s00401-015-1449-5
Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ (2007) Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68(18):1501–1508. doi:10.1212/01.wnl.0000260698.46517.8f
Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, van der Flier WM, Morris JC et al (2015) Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA neurology 72(11):1275–1280. doi:10.1001/jamaneurol.2015.1867
Shankar GM, Walsh DM (2009) Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener 4:48. doi:10.1186/1750-1326-4-48
Numakawa T, Matsumoto T, Adachi N, Yokomaku D, Kojima M, Takei N, Hatanaka H (2001) Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca(2+) and Na(+) in cultured cerebellar neurons. J Neurosci Res 66(1):96–108
Leal G, Afonso PM, Salazar IL, Duarte CB (2015) Regulation of hippocampal synaptic plasticity by BDNF. Brain Res 1621:82–101. doi:10.1016/j.brainres.2014.10.019
Jeon SJ, Bak H, Seo J, Han SM, Lee SH, Han SH, Kwon KJ, Ryu JH et al (2012) Oroxylin A induces BDNF expression on cortical neurons through adenosine A2A receptor stimulation: a possible role in neuroprotection. Biomol Ther (Seoul) 20(1):27–35. doi:10.4062/biomolther.2012.20.1.027
Jeon SJ, Rhee SY, Seo JE, Bak HR, Lee SH, Ryu JH, Cheong JH, Shin CY et al (2011) Oroxylin A increases BDNF production by activation of MAPK-CREB pathway in rat primary cortical neuronal culture. Neurosci Res 69(3):214–222. doi:10.1016/j.neures.2010.11.008
Tsai YW, Yang YR, Sun SH, Liang KC, Wang RY (2013) Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cereb Blood Flow Metab 33(5):764–773. doi:10.1038/jcbfm.2013.15
Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY (2005) Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci 25(49):11288–11299. doi:10.1523/JNEUROSCI.2284-05.2005
Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, Mahmood A, Zhou D et al (2008) Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma 25(2):130–139. doi:10.1089/neu.2007.0369
Ramirez-Rodriguez G, Ocana-Fernandez MA, Vega-Rivera NM, Torres-Perez OM, Gomez-Sanchez A, Estrada-Camarena E, Ortiz-Lopez L (2014) Environmental enrichment induces neuroplastic changes in middle age female Balb/c mice and increases the hippocampal levels of BDNF, p-Akt and p-MAPK1/2. Neuroscience 260:158–170. doi:10.1016/j.neuroscience.2013.12.026
Kozisek ME, Middlemas D, Bylund DB (2008) The differential regulation of BDNF and TrkB levels in juvenile rats after four days of escitalopram and desipramine treatment. Neuropharmacology 54(2):251–257. doi:10.1016/j.neuropharm.2007.08.001
Rantamaki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A, Lesch KP, Rios M et al (2011) Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS One 6(6):e20567. doi:10.1371/journal.pone.0020567
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (91232707, 81271218), National High-Tech R&D Program (863 Program) (No. 2015AA020508), and Natural Science Foundation of Jiangsu Province (No. BK2012746).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were reviewed and approved by the international guidelines for the ethical use of laboratory animals and the Animal Care and Use Committee of Southeast University.
Rights and permissions
About this article
Cite this article
Gong, WG., Wang, YJ., Zhou, H. et al. Citalopram Ameliorates Synaptic Plasticity Deficits in Different Cognition-Associated Brain Regions Induced by Social Isolation in Middle-Aged Rats. Mol Neurobiol 54, 1927–1938 (2017). https://doi.org/10.1007/s12035-016-9781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9781-x