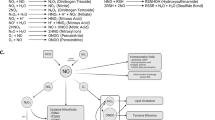
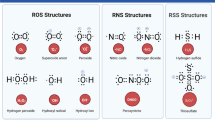
Abstract
Parkinson’s disease (PD) is the second most common neurodegenerative disease. The major characteristics of PD include the loss of dopaminergic neurons in the substantia nigra and Lewy body depositions. Genetic defects, environment toxicants, and aging have been recognized as risk factors for the development of PD. Currently, although the pathogenesis of PD is still obscure, overwhelming evidence demonstrates that oxidative stress plays a central role in the progress of PD. Reactive oxygen species (ROS) function mainly through chemical reactions with atomic targets that lead to covalent oxidative modifications. Through the oxidative modification of ions, amino acids, amines, and nucleic acids, ROS exert augmented effects on the structures and functions of multiple macromolecules. These oxidative modifications can affect nucleic acid stability by oxidizing RNA, increasing mitochondrial DNA (mtDNA) mutation, and launching translesion synthesis (TLS); disturb protein homeostasis by accelerating α-synuclein aggregation, parkin aggregation, and proteasome dissociation; modulate dopamine release by activating ATP-sensitive potassium channels (KATP) and inactivating neuronal nicotinic acetylcholine receptors (nAChRs); and influence cellular self-defenses by promoting the cytoprotective effects of DJ-1 and PTEN-induced putative kinase 1 (PINK1) while inducing Akt dysregulation. Based on the above facts, we propose that various oxidative modifications may affect nucleic acid stability, protein homeostasis, the functionality of ion channels, and cellular self-defenses and that these processes lead to protein misfolding, dopamine depletion, and further neuronal death in PD.






Similar content being viewed by others
Reference
Mayeux R, Stern Y, Rosen J, Leventhal J (1981) Depression, intellectual impairment, and Parkinson disease. Neurology 31(6):645–650
Greenamyre JT, Hastings TG (2004) Parkinson’s: divergent causes, convergent mechanisms. Science 304(5674):1120–1122
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276(5321):2045–2047
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT et al (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2):106–108. doi:10.1038/ng0298-106
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J et al (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173. doi:10.1002/ana.10795
International Parkinson Disease Genomics C, Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simon-Sanchez J, Schulte C, Lesage S, Sveinbjornsdottir S, Stefansson K, Martinez M, Hardy J, Heutink P, Brice A, Gasser T, Singleton AB, Wood NW (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377(9766):641–649. doi:10.1016/S0140-6736(10)62345-8
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E et al (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46(9):989–993. doi:10.1038/ng.3043
Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C (2010) Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat 31(7):763–780. doi:10.1002/humu.21277
Cruts M, Theuns J, Van Broeckhoven C (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat 33(9):1340–1344. doi:10.1002/humu.22117
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535. doi:10.1016/S1474-4422(06)70471-9
Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR (1999) Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology 53(8):1787–1793
Zapico SC, Ubelaker DH (2013) mtDNA mutations and their role in aging, diseases and forensic sciences. Aging Dis 4(6):364–380
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392(6676):605–608. doi:10.1038/33416
Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ et al (1998) The ubiquitin pathway in Parkinson’s disease. Nature 395(6701):451–452. doi:10.1038/26652
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F et al (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299(5604):256–259. doi:10.1126/science.1077209
Shen J, Cookson MR (2004) Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron 43(3):301–304. doi:10.1016/j.neuron.2004.07.012
Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci : CMLS 65(7–8):1272–1284. doi:10.1007/s00018-008-7589-1
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Park Dis 3(4):461–491. doi:10.3233/JPD-130230
Liu M, Bing G (2011) Lipopolysaccharide animal models for Parkinson’s disease. Park Dis 2011:327089. doi:10.4061/2011/327089
Drechsel DA, Patel M (2008) Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med 44(11):1873–1886. doi:10.1016/j.freeradbiomed.2008.02.008
Gao HM, Hong JS (2011) Gene-environment interactions: key to unraveling the mystery of Parkinson’s disease. Prog Neurobiol 94(1):1–19. doi:10.1016/j.pneurobio.2011.03.005
Reeve A, Simcox E, Turnbull D (2014) Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev 14:19–30
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Kudo H, Kokunai T, Kondoh T, Tamaki N, Matsumoto S (1990) Quantitative analysis of glutathione in rat central nervous system: comparison of GSH in infant brain with that in adult brain. Brain Res 511(2):326–328
Das KC, White CW (2002) Redox systems of the cell: possible links and implications. Proc Natl Acad Sci U S A 99(15):9617–9618. doi:10.1073/pnas.162369199
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8(9–10):1865–1879. doi:10.1089/ars.2006.8.1865
Ghosh D, LeVault KR, Barnett AJ, Brewer GJ (2012) A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons. J Neurosci 32(17):5821–5832. doi:10.1523/Jneurosci.6192-11.2012
Sian J, Dexter DT, Lees AJ, Daniel S, Jenner P, Marsden CD (1994) Glutathione-related enzymes in brain in Parkinson’s disease. Ann Neurol 36(3):356–361. doi:10.1002/ana.410360306
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25. doi:10.1016/j.freeradbiomed.2013.05.001
von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J (2015) Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7:124. doi:10.3389/fnagi.2015.00124
Qin L, Liu Y, Hong JS, Crews FT (2013) NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61(6):855–868. doi:10.1002/glia.22479
Yang CM, Hsieh HL, Lin CC, Shih RH, Chi PL, Cheng SE, Hsiao LD (2013) Multiple factors from bradykinin-challenged astrocytes contribute to the neuronal apoptosis: involvement of astroglial ROS, MMP-9, and HO-1/CO system. Mol Neurobiol 47(3):1020–1033. doi:10.1007/s12035-013-8402-1
Danielson SR, Andersen JK (2008) Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radic Biol Med 44(10):1787–1794. doi:10.1016/j.freeradbiomed.2008.03.005
Szabo-Taylor K, Ryan B, Osteikoetxea X, Szabo TG, Sodar B, Holub M, Nemeth A, Paloczi K et al (2015) Oxidative and other posttranslational modifications in extracellular vesicle biology. Semin Cell Dev Biol 40:8–16. doi:10.1016/j.semcdb.2015.02.012
Farooqui AA, Ong W-Y, Horrocks LA (2008) Neurochemical aspects of excitotoxicity. Springer
Farooqui AA (2011) Neurochemical aspects in inflammation in brain. Molecular Aspects of Neurodegeneration and Neuroprotection:13–29
Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R (2011) NOX2 complex-derived ROS as immune regulators. Antioxid Redox Signal 15(8):2197–2208
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344. doi:10.1113/jphysiol.2003.049478
Labunskyy VM, Gladyshev VN (2013) Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal 19(12):1362–1372. doi:10.1089/ars.2012.4891
Farooqui AA, Horrocks LA, Farooqui T (2000) Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem Phys Lipids 106(1):1–29. doi:10.1016/S0009-3084(00)00128-6
Farooqui AA, Horrocks LA (2007) Glycerophospholipids in the brain: phospholipases A2 in neurological disorders. Springer
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13(5):349–361. doi:10.1038/nri3423
D’Autreaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Bio 8(10):813–824. doi:10.1038/Nrm2256
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. doi:10.1152/physrev.00018.2001
Burhans WC, Heintz NH (2009) The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med 47(9):1282–1293. doi:10.1016/j.freeradbiomed.2009.05.026
Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K (2006) Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ 13(5):730–737
Fimognari C (2015) Role of oxidative RNA damage in chronic-degenerative diseases. Oxid Med Cell Longev 2015:358713. doi:10.1155/2015/358713
Tanaka M, Chock PB, Stadtman ER (2007) Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci U S A 104(1):66–71. doi:10.1073/pnas.0609737104
Deleault NR, Lucassen RW, Supattapone S (2003) RNA molecules stimulate prion protein conversion. Nature 425(6959):717–720. doi:10.1038/nature01979
Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ (1999) Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol 154(5):1423–1429. doi:10.1016/S0002-9440(10)65396-5
Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, Nunomura A, Castellani RJ et al (2002) Systemic increase of oxidative nucleic acid damage in Parkinson’s disease and multiple system atrophy. Neurobiol Dis 9(2):244–248. doi:10.1006/nbdi.2002.0466
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419(6903):135–141. doi:10.1038/Nature00991
Woo DK, Green PD, Santos JH, D’Souza AD, Walther Z, Martin WD, Christian BE, Chandel NS et al (2012) Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am J Pathol 180(1):24–31
Enright H, Miller WJ, Hays R, Floyd RA, Hebbel RP (1996) Preferential targeting of oxidative base damage to internucleosomal DNA. Carcinogenesis 17(5):1175–1177
Nohl H, Gille L, Staniek K (2005) Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol 69(5):719–723. doi:10.1016/j.bcp.2004.12.002
Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y et al (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320(5876):661–664
Lagouge M, Larsson NG (2013) The role of mitochondrial DNA mutations and free radicals in disease and ageing. J Intern Med 273(6):529–543. doi:10.1111/joim.12055
Hroudova J, Singh N, Fisar Z (2014) Mitochondrial dysfunctions in neurodegenerative diseases: relevance to Alzheimer’s disease. Biomed Res Int 2014:175062. doi:10.1155/2014/175062
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106:17–32. doi:10.1016/j.pneurobio.2013.04.004
Ekstrand MI, Terzioglu M, Galter D, Zhu SW, Hofstetter C, Lindqvist E, Thams S, Bergstrand A et al (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104(4):1325–1330. doi:10.1073/pnas.0605208103
Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY et al (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309(5733):481–484. doi:10.1126/science.1112125
Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R et al (2005) Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A 102(50):17993–17998. doi:10.1073/pnas.0508886102
Thompson LV (2006) Oxidative stress, mitochondria and mtDNA-mutator mice. Exp Gerontol 41(12):1220–1222. doi:10.1016/j.exger.2006.10.018
Wilkinson KD (1997) Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J 11(14):1245–1256
Reyes-Turcu FE, Ventii KH, Wilkinson KD (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem 78:363–397. doi:10.1146/annurev.biochem.78.082307.091526
Acharya N, Yoon JH, Gali H, Unk I, Haracska L, Johnson RE, Hurwitz J, Prakash L et al (2008) Roles of PCNA-binding and ubiquitin-binding domains in human DNA polymerase eta in translesion DNA synthesis. Proc Natl Acad Sci U S A 105(46):17724–17729. doi:10.1073/pnas.0809844105
Zlatanou A, Despras E, Braz-Petta T, Boubakour-Azzouz I, Pouvelle C, Stewart GS, Nakajima S, Yasui A et al (2011) The hMsh2-hMsh6 complex acts in concert with monoubiquitinated PCNA and Pol eta in response to oxidative DNA damage in human cells. Mol Cell 43(4):649–662. doi:10.1016/j.molcel.2011.06.023
Lee JG, Baek K, Soetandyo N, Ye Y (2013) Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat Commun 4:1568. doi:10.1038/ncomms2532
Watts FZ (2013) Starting and stopping SUMOylation. What regulates the regulator? Chromosoma 122(6):451–463. doi:10.1007/s00412-013-0422-0
Bossis G, Melchior F (2006) Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell 21(3):349–357. doi:10.1016/j.molcel.2005.12.019
Bossis G, Melchior F (2006) SUMO: regulating the regulator. Cell Div 1:13. doi:10.1186/1747-1028-1-13
Hay RT (2005) SUMO: a history of modification. Mol Cell 18(1):1–12. doi:10.1016/j.molcel.2005.03.012
Gali H, Juhasz S, Morocz M, Hajdu I, Fatyol K, Szukacsov V, Burkovics P, Haracska L (2012) Role of SUMO modification of human PCNA at stalled replication fork. Nucleic Acids Res 40(13):6049–6059. doi:10.1093/nar/gks256
Douglas PM, Dillin A (2010) Protein homeostasis and aging in neurodegeneration. J Cell Biol 190(5):719–729. doi:10.1083/jcb.201005144
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. doi:10.1038/42166
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95(11):6469–6473. doi:10.1073/pnas.95.11.6469
Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294(5545):1346–1349. doi:10.1126/science.1063522
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298. doi:10.1146/annurev.neuro.26.010302.081142
Zhou W, Freed CR (2004) Tyrosine-to-cysteine modification of human alpha-synuclein enhances protein aggregation and cellular toxicity. J Biol Chem 279(11):10128–10135. doi:10.1074/jbc.M307563200
Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL (2002) Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett 517(1–3):239–244
Hokenson MJ, Uversky VN, Goers J, Yamin G, Munishkina LA, Fink AL (2004) Role of individual methionines in the fibrillation of methionine-oxidized alpha-synuclein. Biochem Us 43(15):4621–4633. doi:10.1021/Bi049979h
Glaser CB, Yamin G, Uversky VN, Fink AL (2005) Methionine oxidation, alpha-synuclein and Parkinson’s disease. Biochim Biophys Acta 1703(2):157–169. doi:10.1016/j.bbapap.2004.10.008
Leong SL, Pham CL, Galatis D, Fodero-Tavoletti MT, Perez K, Hill AF, Masters CL, Ali FE et al (2009) Formation of dopamine-mediated alpha-synuclein-soluble oligomers requires methionine oxidation. Free Radic Biol Med 46(10):1328–1337. doi:10.1016/j.freeradbiomed.2009.02.009
Leong SL, Cappai R, Barnham KJ, Pham CL (2009) Modulation of alpha-synuclein aggregation by dopamine: a review. Neurochem Res 34(10):1838–1846. doi:10.1007/s11064-009-9986-8
Bisaglia M, Mammi S, Bubacco L (2007) Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 282(21):15597–15605. doi:10.1074/jbc.M610893200
Pham CL, Leong SL, Ali FE, Kenche VB, Hill AF, Gras SL, Barnham KJ, Cappai R (2009) Dopamine and the dopamine oxidation product 5,6-dihydroxylindole promote distinct on-pathway and off-pathway aggregation of alpha-synuclein in a pH-dependent manner. J Mol Biol 387(3):771–785. doi:10.1016/j.jmb.2009.02.007
Bisaglia M, Tosatto L, Munari F, Tessari I, de Laureto PP, Mammi S, Bubacco L (2010) Dopamine quinones interact with alpha-synuclein to form unstructured adducts. Biochem Biophys Res Commun 394(2):424–428. doi:10.1016/j.bbrc.2010.03.044
Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ (2003) The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 37(4):583–595. doi:10.1016/S0896-6273(03)00024-2
Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL (2007) Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem 282(8):5862–5870. doi:10.1074/jbc.M608126200
Kostka M, Hogen T, Danzer KM, Levin J, Habeck M, Wirth A, Wagner R, Glabe CG et al (2008) Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J Biol Chem 283(16):10992–11003. doi:10.1074/jbc.M709634200
Hillmer AS, Putcha P, Levin J, Hogen T, Hyman BT, Kretzschmar H, McLean PJ, Giese A (2010) Converse modulation of toxic alpha-synuclein oligomers in living cells by N′-benzylidene-benzohydrazide derivates and ferric iron. Biochem Biophys Res Commun 391(1):461–466. doi:10.1016/j.bbrc.2009.11.080
Levin J, Hogen T, Hillmer AS, Bader B, Schmidt F, Kamp F, Kretzschmar HA, Botzel K et al (2011) Generation of ferric iron links oxidative stress to alpha-synuclein oligomer formation. J Park Dis 1(2):205–216. doi:10.3233/JPD-2011-11040
Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Iwai K et al (2000) Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25(3):302–305
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40(2):427–446
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479. doi:10.1146/annurev.biochem.67.1.425
Dantuma NP, Bott LC (2014) The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci 7:70. doi:10.3389/fnmol.2014.00070
Meng F, Yao D, Shi Y, Kabakoff J, Wu W, Reicher J, Ma Y, Moosmann B et al (2011) Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener 6:34. doi:10.1186/1750-1326-6-34
Glickman MH, Rubin DM, Coux O, Wefes I, Pfeifer G, Cjeka Z, Baumeister W, Fried VA et al (1998) A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell 94(5):615–623
Davies KJ (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83(3–4):301–310
Bader N, Grune T (2006) Protein oxidation and proteolysis. Biol Chem 387(10–11):1351–1355. doi:10.1515/BC.2006.169
Wang X, Yen J, Kaiser P, Huang L (2010) Regulation of the 26S proteasome complex during oxidative stress. Sci Signal 3(151):ra88. doi:10.1126/scisignal.2001232
Silva GM, Netto LE, Simoes V, Santos LF, Gozzo FC, Demasi MA, Oliveira CL, Bicev RN et al (2012) Redox control of 20S proteasome gating. Antioxid Redox Signal 16(11):1183–1194. doi:10.1089/ars.2011.4210
Livnat-Levanon N, Kevei E, Kleifeld O, Krutauz D, Segref A, Rinaldi T, Erpapazoglou Z, Cohen M et al (2014) Reversible 26S proteasome disassembly upon mitochondrial stress. Cell Rep 7(5):1371–1380. doi:10.1016/j.celrep.2014.04.030
Dragicevic E, Schiemann J, Liss B (2015) Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 284:798–814. doi:10.1016/j.neuroscience.2014.10.037
Quik M, Wonnacott S (2011) alpha6beta2* and alpha4beta2* nicotinic acetylcholine receptors as drug targets for Parkinson’s disease. Pharmacol Rev 63(4):938–966
Kourie JI (1998) Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol 275(1 Pt 1):C1–C24
Horenstein J, Wagner DA, Czajkowski C, Akabas MH (2001) Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat Neurosci 4(5):477–485
Campanucci VA, Krishnaswamy A, Cooper E (2008) Mitochondrial reactive oxygen species inactivate neuronal nicotinic acetylcholine receptors and induce long-term depression of fast nicotinic synaptic transmission. J Neurosci 28(7):1733–1744. doi:10.1523/JNEUROSCI.5130-07.2008
Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) The open pore conformation of potassium channels. Nature 417(6888):523–526. doi:10.1038/417523a
Enkvetchakul D, Nichols CG (2003) Gating mechanism of KATP channels: function fits form. J Gen Physiol 122(5):471–480. doi:10.1085/jgp.200308878
Drain P, Geng X, Li L (2004) Concerted gating mechanism underlying KATP channel inhibition by ATP. Biophys J 86(4):2101–2112. doi:10.1016/S0006-3495(04)74269-1
Sun HS, Feng ZP (2013) Neuroprotective role of ATP-sensitive potassium channels in cerebral ischemia. Acta Pharmacol Sin 34(1):24–32. doi:10.1038/aps.2012.138
Wilson CJ (1993) The generation of natural firing patterns in neostriatal neurons. Prog Brain Res 99:277–297
Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994) Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14(10):6084–6093
Avshalumov MV, Patel JC, Rice ME (2008) AMPA receptor-dependent H2O2 generation in striatal medium spiny neurons but not dopamine axons: one source of a retrograde signal that can inhibit dopamine release. J Neurophysiol 100(3):1590–1601
Babenko AP, Aguilar-Bryan L, Bryan J (1998) A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60:667–687. doi:10.1146/annurev.physiol.60.1.667
Miki T, Nagashima K, Seino S (1999) The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic beta-cells. J Mol Endocrinol 22(2):113–123
Seino S, Miki T (2003) Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81(2):133–176
Avshalumov MV, Chen BT, Marshall SP, Pena DM, Rice ME (2003) Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J Neurosci 23(7):2744–2750
Tanner CM (2010) Advances in environmental epidemiology. Mov Disord 25(Suppl 1):S58–S62. doi:10.1002/mds.22721
Ryan RE, Ross SA, Drago J, Loiacono RE (2001) Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol 132(8):1650–1656. doi:10.1038/sj.bjp.0703989
Srinivasan R, Henderson BJ, Lester HA, Richards CI (2014) Pharmacological chaperoning of nAChRs: a therapeutic target for Parkinson’s disease. Pharmacol Res 83:20–29. doi:10.1016/j.phrs.2014.02.005
Mazzo F, Pistillo F, Grazioso G, Clementi F, Borgese N, Gotti C, Colombo SF (2013) Nicotine-modulated subunit stoichiometry affects stability and trafficking of alpha3beta4 nicotinic receptor. J Neurosci 33(30):12316–12328. doi:10.1523/JNEUROSCI.2393-13.2013
Krishnaswamy A, Cooper E (2012) Reactive oxygen species inactivate neuronal nicotinic acetylcholine receptors through a highly conserved cysteine near the intracellular mouth of the channel: implications for diseases that involve oxidative stress. J Physiol 590(1):39–47
Dajas-Bailador F, Wonnacott S (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25(6):317–324. doi:10.1016/j.tips.2004.04.006
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M et al (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23(21):7820–7829
Campanucci V, Krishnaswamy A, Cooper E (2010) Diabetes depresses synaptic transmission in sympathetic ganglia by inactivating nAChRs through a conserved intracellular cysteine residue. Neuron 66(6):827–834. doi:10.1016/j.neuron.2010.06.010
Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N et al (2008) In vivo activation of midbrain dopamine neurons via sensitized, high-affinity a6* nicotinic acetylcholine receptors. Neuron 60(1):123–136
Quik M, Perez XA, Bordia T (2012) Nicotine as a potential neuroprotective agent for Parkinson’s disease. Mov Disord 27(8):947–957. doi:10.1002/Mds.25028
Waak J, Weber SS, Gorner K, Schall C, Ichijo H, Stehle T, Kahle PJ (2009) Oxidizable residues mediating protein stability and cytoprotective interaction of DJ-1 with apoptosis signal-regulating kinase 1. J Biol Chem 284(21):14245–14257. doi:10.1074/jbc.M806902200
Wilson MA (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal 15(1):111–122. doi:10.1089/ars.2010.3481
Shinbo Y, Niki T, Taira T, Ooe H, Takahashi-Niki K, Maita C, Seino C, Iguchi-Ariga SMM et al (2006) Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ 13(1):96–108. doi:10.1038/sj.cdd.4401704
Ariga H, Takahashi-Niki K, Kato I, Maita H, Niki T, Iguchi-Ariga SMM (2013) Neuroprotective function of DJ-1 in Parkinson’s disease. Oxidative Med Cell Longev. doi:10.1155/2013/683920, Unsp 683920
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D et al (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304(5674):1158–1160. doi:10.1126/science.1096284
Chien WL, Lee TR, Hung SY, Kang KH, Wu RM, Lee MJ, Fu WM (2013) Increase of oxidative stress by a novel PINK1 mutation, P209A. Free Radic Biol Med 58:160–169. doi:10.1016/j.freeradbiomed.2012.12.008
Chien WL, Lee TR, Hung SY, Kang KH, Lee MJ, Fu WM (2011) Impairment of oxidative stress-induced heme oxygenase-1 expression by the defect of Parkinson-related gene of PINK1. J Neurochem 117(4):643–653. doi:10.1111/j.1471-4159.2011.07229.x
Malagelada C, Jin ZH, Greene LA (2008) RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J Neurosci 28(53):14363–14371. doi:10.1523/Jneurosci.3928-08.2008
Durgadoss L, Nidadavolu P, Valli RK, Saeed U, Mishra M, Seth P, Ravindranath V (2012) Redox modification of Akt mediated by the dopaminergic neurotoxin MPTP, in mouse midbrain, leads to down-regulation of pAkt. FASEB J 26(4):1473–1483. doi:10.1096/fj.11-194100
Parkinson Study Group QEI, Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, Juncos JL et al (2014) A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol 71(5):543–552. doi:10.1001/jamaneurol.2014.131
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31571202, 31271136, and 81371398), the Young Talents Cultivation Plan in Beijing Local University (CIT and TCD201504087), the Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201210025020 and 7131001), and the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140514).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, J., Yu, S., Zheng, Y. et al. Oxidative Modification and Its Implications for the Neurodegeneration of Parkinson’s Disease. Mol Neurobiol 54, 1404–1418 (2017). https://doi.org/10.1007/s12035-016-9743-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9743-3