Abstract
Mesenchymal stem cells (MSCs) have recently been described to home to brain tumors and to integrate into the tumor-associated stroma. Understanding the communication between cancer cells and MSCs has become fundamental to determine whether MSC-tumor interactions should be exploited as a vehicle for therapeutic agents or considered a target for intervention. Therefore, we investigated whether conditioned medium from adipose-derived stem cells (ADSCs-CM) modulate glioma tumor cells by analyzing several cell biology processes in vitro. C6 rat glioma cells were treated with ADSCs-CM, and cell proliferation, cell cycle, cell viability, cell morphology, adhesion, migration, and expression of epithelial-mesenchymal transition (EMT)-related surface markers were analyzed. ADSCs-CM did not alter cell viability, cell cycle, and growth rate of C6 glioma cells but increased their migratory capacity. Moreover, C6 cells treated with ADSC-CM showed reduced adhesion and underwent changes in cell morphology. Up-regulation of EMT-associated markers (vimentin, MMP2, and NRAS) was also observed following treatment with ADSC-CM. Our findings demonstrate that the paracrine factors released by ADSCs are able to modulate glioma cell biology. Therefore, ADSC-tumor cell interactions in a tumor microenvironment must be considered in the design of clinical application of stem cell therapy.

Factors released by adipose-derived stem cells (ADSCs) may modulate the biology of C6 glioma cells. When C6 cells are exposed to a conditioned medium from adipose-derived stem cells (ADSCs-CM), some of these cells can undergo an EMT-like process and trans-differentiate into cells with a more mesenchymal phenotype, characterized by enhanced expression of EMT-related surface markers, reduced cell adhesion capacity, increased migratory capacity, as well as changes in cell and nuclei morphology.







Similar content being viewed by others
References
Becker KP, Yu J (2012) Status quo—standard-of-care medical and radiation therapy for glioblastoma. Cancer J 18(1):12–19. doi:10.1097/PPO.0b013e318244d7eb
Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, Garcia C, Romao L et al (2012) Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 1826(2):338–349. doi:10.1016/j.bbcan.2012.05.004
Westphal M, Lamszus K (2011) The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci 12(9):495–508. doi:10.1038/nrn3060
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A 97(12):6242–6244
Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G (2013) Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol 23(6 Pt B):522–532. doi:10.1016/j.semcancer.2013.08.007
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315(26):1650–1659. doi:10.1056/NEJM198612253152606
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, Miller N, Hennessy E et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124(2):317–326. doi:10.1007/s10549-010-0734-1
Chagastelles PC, Nardi NB, Camassola M (2010) Biology and applications of mesenchymal stem cells. Sci Prog 93(Pt 2):113–127
Meirelles Lda S, Fontes AM, Covas DT, Caplan AI (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20(5–6):419–427. doi:10.1016/j.cytogfr.2009.10.002
Glaser T, Cappellari AR, Pillat MM, Iser IC, Wink MR, Battastini AM, Ulrich H (2012) Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal 8(3):523–537. doi:10.1007/s11302-011-9282-3
Iser IC, Bracco PA, Goncalves CE, Zanin RF, Nardi NB, Lenz G, Battastini AM, Wink MR (2014) Mesenchymal stem cells from different murine tissues have differential capacity to metabolize extracellular nucleotides. J Cell Biochem 115(10):1673–1682. doi:10.1002/jcb.24830
Altaner C, Altanerova V (2012) Stem cell based glioblastoma gene therapy. Neoplasma 59(6):756–760. doi:10.4149/neo_2012_95
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S et al (2005) Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65(8):3307–3318. doi:10.1158/0008-5472.CAN-04-1874
Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C (2007) Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res 67(13):6304–6313. doi:10.1158/0008-5472.CAN-06-4024
Bak XY, Lam DH, Yang J, Ye K, Wei EL, Lim SK, Wang S (2011) Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther 22(11):1365–1377. doi:10.1089/hum.2010.212
Chien LY, Hsiao JK, Hsu SC, Yao M, Lu CW, Liu HM, Chen YC, Yang CS, Huang DM In vivo magnetic resonance imaging of cell tropism, trafficking mechanism, and therapeutic impact of human mesenchymal stem cells in a murine glioma model. Biomaterials 32(12):3275–3284. doi: 10.1016/j.biomaterials.2011.01.042
Bianchi G, Morandi F, Cilli M, Daga A, Bocelli-Tyndall C, Gambini C, Pistoia V, Raffaghello L (2012) Close interactions between mesenchymal stem cells and neuroblastoma cell lines lead to tumor growth inhibition. PLoS ONE 7(10):e48654. doi:10.1371/journal.pone.0048654
Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G et al (2008) VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer 99(4):622–631. doi:10.1038/sj.bjc.6604508
De Luca A, Lamura L, Gallo M, Maffia V, Normanno N Mesenchymal stem cell-derived interleukin-6 and vascular endothelial growth factor promote breast cancer cell migration. J Cell Biochem 113 (11):3363–3370. doi:10.1002/jcb.24212
Kang SG, Jeun SS, Lim JY, Kim SM, Yang YS, Oh WI, Huh PW, Park CK (2008) Cytotoxicity of human umbilical cord blood-derived mesenchymal stem cells against human malignant glioma cells. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 24(3):293–302. doi:10.1007/s00381-007-0515-2
Chien LY, Hsiao JK, Hsu SC, Yao M, Lu CW, Liu HM, Chen YC, Yang CS et al (2011) In vivo magnetic resonance imaging of cell tropism, trafficking mechanism, and therapeutic impact of human mesenchymal stem cells in a murine glioma model. Biomaterials 32(12):3275–3284. doi:10.1016/j.biomaterials.2011.01.042
Jiao H, Guan F, Yang B, Li J, Shan H, Song L, Hu X, Du Y (2011) Human umbilical cord blood-derived mesenchymal stem cells inhibit C6 glioma via downregulation of cyclin D1. Neurol India 59(2):241–247. doi:10.4103/0028-3886.79134
Pisati F, Belicchi M, Acerbi F, Marchesi C, Giussani C, Gavina M, Javerzat S, Hagedorn M et al (2007) Effect of human skin-derived stem cells on vessel architecture, tumor growth, and tumor invasion in brain tumor animal models. Cancer Res 67(7):3054–3063. doi:10.1158/0008-5472.CAN-06-1384
Liu J, Zhang Y, Bai L, Cui X, Zhu J (2012) Rat bone marrow mesenchymal stem cells undergo malignant transformation via indirect co-cultured with tumour cells. Cell Biochem Funct 30(8):650–656. doi:10.1002/cbf.2844
Yu JM, Jun ES, Bae YC, Jung JS (2008) Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev 17(3):463–473. doi:10.1089/scd.2007.0181
Behnan J, Isakson P, Joel M, Cilio C, Langmoen IA, Vik-Mo EO, Badn W (2014) Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells 32(5):1110–1123. doi:10.1002/stem.1614
Ochs K, Sahm F, Opitz CA, Lanz TV, Oezen I, Couraud PO, von Deimling A, Wick W et al (2013) Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol 265(1–2):106–116. doi:10.1016/j.jneuroim.2013.09.011
Akimoto K, Kimura K, Nagano M, Takano S, To’a Salazar G, Yamashita T, Ohneda O (2013) Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev 22(9):1370–1386. doi:10.1089/scd.2012.0486
Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P (2013) Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta 1836(2):321–335. doi:10.1016/j.bbcan.2013.10.004
Kahlert UD, Nikkhah G, Maciaczyk J (2013) Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett 331(2):131–138. doi:10.1016/j.canlet.2012.12.010
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428. doi:10.1172/JCI39104
Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y, Funakoshi S, Adachi M et al (2013) Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci 104(2):157–164. doi:10.1111/cas.12059
Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S et al (2013) Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4:1795. doi:10.1038/ncomms2766
Jing Y, Han Z, Liu Y, Sun K, Zhang S, Jiang G, Li R, Gao L et al (2012) Mesenchymal stem cells in inflammation microenvironment accelerates hepatocellular carcinoma metastasis by inducing epithelial-mesenchymal transition. PLoS ONE 7(8):e43272. doi:10.1371/journal.pone.0043272
Xu Q, Wang L, Li H, Han Q, Li J, Qu X, Huang S, Zhao RC (2012) Mesenchymal stem cells play a potential role in regulating the establishment and maintenance of epithelial-mesenchymal transition in MCF7 human breast cancer cells by paracrine and induced autocrine TGF-beta. Int J Oncol 41(3):959–968. doi:10.3892/ijo.2012.1541
da Silva Meirelles L, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119(Pt 11):2204–2213
Overton WR, McCoy JP Jr (1994) Reversing the effect of formalin on the binding of propidium iodide to DNA. Cytometry 16(4):351–356. doi:10.1002/cyto.990160410
Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z et al (2008) Ecto-5’-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol 134(3):365–372. doi:10.1007/s00432-007-0292-z
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2(2):329–333. doi:10.1038/nprot.2007.30
Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, Lucci A, Singh B et al (2009) Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 15(21):6639–6648. doi:10.1158/1078-0432.CCR-09-0951
Zhan R, Yang S, He W, Wang F, Tan J, Zhou J, Yang S, Yao Z et al (2015) Nitric oxide enhances keratinocyte cell migration by regulating Rho GTPase via cGMP-PKG signalling. PLoS ONE 10(3):e0121551. doi:10.1371/journal.pone.0121551
Filippi-Chiela EC, Oliveira MM, Jurkovski B, Callegari-Jacques SM, da Silva VD, Lenz G (2012) Nuclear morphometric analysis (NMA): screening of senescence, apoptosis and nuclear irregularities. PLoS ONE 7(8):e42522. doi:10.1371/journal.pone.0042522
Iser IC CR, Bertoni APS, Wink MR (2015) Identification of valid endogenous control genes for determining gene expression in C6 glioma cell line treated with conditioned medium from adipose-derived stem cell. Biomed Pharmacother 75:75–82. doi:10.1016/j.biopha.2015.08.035
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S, Diez-Tejedor E (2013) Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 4(1):11. doi:10.1186/scrt159
Shirkoohi R (2013) Epithelial mesenchymal transition from a natural gestational orchestration to a bizarre cancer disturbance. Cancer Sci 104(1):28–35. doi:10.1111/cas.12074
Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC et al (2012) EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci CMLS 69(20):3429–3456. doi:10.1007/s00018-012-1122-2
Kucerova L, Skolekova S, Matuskova M, Bohac M, Kozovska Z (2013) Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer 13:535. doi:10.1186/1471-2407-13-535
Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R et al (2003) Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res 63(8):1969–1974
Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, Alt EU (2010) Adipose tissue-derived stem cells promote prostate tumor growth. Prostate 70(15):1709–1715. doi:10.1002/pros.21206
Bhattacharya SD, Mi Z, Talbot LJ, Guo H, Kuo PC (2012) Human mesenchymal stem cell and epithelial hepatic carcinoma cell lines in admixture: concurrent stimulation of cancer-associated fibroblasts and epithelial-to-mesenchymal transition markers. Surgery 152(3):449–454. doi:10.1016/j.surg.2012.06.011
Hong IS, Lee HY, Kang KS (2014) Mesenchymal stem cells and cancer: friends or enemies? Mutat Res 768:98–106. doi:10.1016/j.mrfmmm.2014.01.006
Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD (2013) Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 95(12):2235–2245. doi:10.1016/j.biochi.2013.05.010
Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X et al (2011) Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle 10(18):3198–3207
Makridakis M, Roubelakis MG, Vlahou A (2013) Stem cells: insights into the secretome. Biochim Biophys Acta 1834(11):2380–2384. doi:10.1016/j.bbapap.2013.01.032
Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS et al (2008) Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE 3(6):e2563. doi:10.1371/journal.pone.0002563
Xue Z, Wu X, Chen X, Liu Y, Wang X, Wu K, Nie Y, Fan D (2015) Mesenchymal stem cells promote epithelial to mesenchymal transition and metastasis in gastric cancer though paracrine cues and close physical contact. J Cell Biochem 116(4):618–627. doi:10.1002/jcb.25013
Li X, Luo Q, Sun J, Song G (2015) Conditioned medium from mesenchymal stem cells enhances the migration of hepatoma cells through CXCR4 up-regulation and F-actin remodeling. Biotechnol Lett 37(3):511–521. doi:10.1007/s10529-014-1710-3
Yang X, Li Z, Ma Y, Gao J, Liu S, Gao Y, Wang G (2014) Human umbilical cord mesenchymal stem cells promote carcinoma growth and lymph node metastasis when co-injected with esophageal carcinoma cells in nude mice. Cancer Cell Int 14(1):93. doi:10.1186/s12935-014-0093-9
Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, Tanaka S, Yasui W et al (2010) Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer 127(10):2323–2333. doi:10.1002/ijc.25440
Wang M, Wang T, Liu S, Yoshida D, Teramoto A (2003) The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol 20(2):65–72
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563. doi:10.1038/nature06188
Fonkem E, Lun M, Wong ET (2011) Rare phenomenon of extracranial metastasis of glioblastoma. J Clin Oncol Off J Am Soc Clin Oncol 29(34):4594–4595. doi:10.1200/JCO.2011.39.0187
Lombard A, Goffart N, Rogister B (2015) Glioblastoma circulating cells: reality, trap or illusion? Stem Cells Int 2015:182985. doi:10.1155/2015/182985
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL et al (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21(21):2683–2710. doi:10.1101/gad.1596707
Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M et al (2015) Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. doi:10.1016/j.semcancer.2015.03.008
Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M (2007) Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs 185(1–3):191–203. doi:10.1159/000101320
Cavallaro U, Christofori G (2001) Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta 1552(1):39–45
Lamouille S, Subramanyam D, Blelloch R, Derynck R (2013) Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol 25(2):200–207. doi:10.1016/j.ceb.2013.01.008
Zhang Q, Helfand BT, Jang TL, Zhu LJ, Chen L, Yang XJ, Kozlowski J, Smith N et al (2009) Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res Off J Am Assoc Cancer Res 15(10):3557–3567. doi:10.1158/1078-0432.CCR-08-1656
McInroy L, Maatta A (2007) Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun 360(1):109–114. doi:10.1016/j.bbrc.2007.06.036
Hendrix MJ, Seftor EA, Chu YW, Seftor RE, Nagle RB, McDaniel KM, Leong SP, Yohem KH et al (1992) Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst 84(3):165–174
Kidd ME, Shumaker DK, Ridge KM (2014) The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50(1):1–6. doi:10.1165/rcmb.2013-0314TR
Toiyama Y, Yasuda H, Saigusa S, Tanaka K, Inoue Y, Goel A, Kusunoki M (2013) Increased expression of slug and vimentin as novel predictive biomarkers for lymph node metastasis and poor prognosis in colorectal cancer. Carcinogenesis 34(11):2548–2557. doi:10.1093/carcin/bgt282
Hamasaki T, Hattori T, Kimura G, Nakazawa N (1998) Tumor progression and expression of matrix metalloproteinase-2 (MMP-2) mRNA by human urinary bladder cancer cells. Urol Res 26(6):371–376
Hofmann UB, Westphal JR, Waas ET, Zendman AJ, Cornelissen IM, Ruiter DJ, van Muijen GN (1999) Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer 81(5):774–782. doi:10.1038/sj.bjc.6690763
Trudel D, Fradet Y, Meyer F, Harel F, Tetu B (2003) Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res 63(23):8511–8515
Durlik M, Gardian K (2012) Metalloproteinase 2 and 9 activity in the development of pancreatic cancer. Pol Przegl Chir 84(8):377–382. doi:10.2478/v10035-012-0064-6
Chintala SK, Tonn JC, Rao JS (1999) Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci Off J Int Soc Dev Neurosci 17(5–6):495–502
Knobbe CB, Reifenberger J, Reifenberger G (2004) Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol 108(6):467–470. doi:10.1007/s00401-004-0929-9
Bleeker FE, Lamba S, Rodolfo M, Scarpa A, Leenstra S, Vandertop WP, Bardelli A (2009) Mutational profiling of cancer candidate genes in glioblastoma, melanoma and pancreatic carcinoma reveals a snapshot of their genomic landscapes. Hum Mutat 30(2):E451–E459. doi:10.1002/humu.20927
Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J et al (2013) A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 24(4):466–480. doi:10.1016/j.ccr.2013.08.018
Horiguchi K, Shirakihara T, Nakano A, Imamura T, Miyazono K, Saitoh M (2009) Role of Ras signaling in the induction of snail by transforming growth factor-beta. J Biol Chem 284(1):245–253. doi:10.1074/jbc.M804777200
Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66(17):8319–8326. doi:10.1158/0008-5472.CAN-06-0410
Strauss R, Hamerlik P, Lieber A, Bartek J (2012) Regulation of stem cell plasticity: mechanisms and relevance to tissue biology and cancer. Mol Ther J Am Soc Gene Ther 20(5):887–897. doi:10.1038/mt.2012.2
Scheel C, Onder T, Karnoub A, Weinberg RA (2007) Adaptation versus selection: the origins of metastatic behavior. Cancer Res 67(24):11476–11479. doi:10.1158/0008-5472.CAN-07-1653, discussion 11479–11480
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437. doi:10.1038/nm.3394
Hombauer H, Minguell JJ (2000) Selective interactions between epithelial tumour cells and bone marrow mesenchymal stem cells. Br J Cancer 82(7):1290–1296. doi:10.1054/bjoc.1999.1093
Velpula KK, Dasari VR, Tsung AJ, Dinh DH, Rao JS (2011) Cord blood stem cells revert glioma stem cell EMT by down regulating transcriptional activation of Sox2 and Twist1. Oncotarget 2(12):1028–1042
Acknowledgments
The authors would like to thank Marília Remuzzi Zandoná (Laboratório de Análises Clínicas, UFCSPA) for the excellent technical assistance with LDH analysis. This work was supported by the Conselho de Desenvolvimento Científico e Tecnológico (CNPq-Brasil) (Edital Universal 475882/2012-1 and Novas Terapias Portadoras de Futuro 457394/2013-7); Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) (Edital Pronem 11/2072-2); and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Brasil) (Edital Probitec 004/2012). I.C. Iser and A.P.S. Bertoni are recipients of CAPES PhD and PNPD-Pos-doc fellowship, respectively. M.R. Wink and G. Lenz are recipients of CNPq research productivity fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
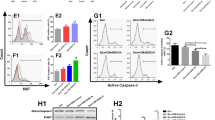
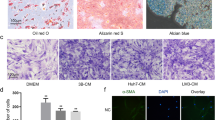
ADSC characterization. The differentiation of ADSCs is shown in light microscopy. A. Immunophenotyping analysis of surface markers expression in ADCSs. Flow cytometry histograms show the expression (bold line) of selected molecules (CD90, CD29, CD11b and CD45) by ADSC populations in comparison with controls. B. ADSCs differentiated into adipocyte-like cells, which was stained by Oil Red O, the triglyceride specific dye. C. Cells differentiated into osteoblasts showing the bone matrix stained by Alizarin Red, the calcium-specific marker. D. Cells differentiated into chondrocytes showing the proteoglycan rich matrix stained by Alcian blue. B and D: Magnification, X 400. C: Magnifications, X 200 (GIF 595 kb)
Rights and permissions
About this article
Cite this article
Iser, I.C., Ceschini, S.M., Onzi, G.R. et al. Conditioned Medium from Adipose-Derived Stem Cells (ADSCs) Promotes Epithelial-to-Mesenchymal-Like Transition (EMT-Like) in Glioma Cells In vitro. Mol Neurobiol 53, 7184–7199 (2016). https://doi.org/10.1007/s12035-015-9585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9585-4