Abstract
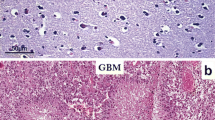
Matrix metalloproteinases (MMPs) have been implicated to play a critical role in glioma invasiveness. In this study, we aimed to investigate, the expression of MMP-2 and MMP-9 in human gliomas of different degrees of malignancy, and evaluated the correlation between MMP-2 and MMP-9 expression in gliomas. The samples from 65 cases of glioma were divided into four groups according to the WHO classification: there were 16 cases of grade I, 17 cases of grade II, 20 cases of grade III, and 12 cases of grade IV. Normal brain samples served as the control group, and biopsy specimens were obtained from 8 glioma patients with a needle placed into the adjacent brain 1 cm from the margin after tumor resection. All the samples were stained with hematoxylin and eosin and immunohistochemistry. A computer-aided image-analysis system was employed to measure the integral optical density (IOD) of positive slides. No positive staining was found in the control group. The positive staining was localized in the cytoplasm of glioma cells, the extracellular matrix (ECM), the basement membrane (BM), and the endothelial cells of blood vessels. Positive staining rates increased significantly when the degree of malignancy of gliomas was elevated. The IOD value of MMP-2 and MMP-9 also indicated that the intensity of MMP-2 and MMP-9 expression was elevated significantly with the degree of malignancy of the gliomas. There was a positive correlation between MMP-2 and MMP-9 expression in gliomas. Glioma invasion and angiogenesis were particularly seen in the biopsied tissues, and MMP-9 immunostaining seemed to be much more intense and extensive than MMP-2 immunostaining in these samples. These results suggest that MMP-2 and MMP-9 staining in gliomas is localized in the cytoplasm of tumor cells, BM, and endothelial cells, and that MMP-2 and MMP-9 together play an important role in the invasiveness of gliomas, mediating the degradation of the ECM and angiogenesis. MMP-2 and MMP-9 could be molecular targets in the treatment of malignant glioma.
Similar content being viewed by others
References
Yoshida D, Piepmeier JM, Bergenheim T, et al (1998) Suppression of metalloproteinase-2 mediated cell invasion in U87MG, human glioma cells by anti-microtubule agent: in vitro study. Br J Cancer 77:21–25
Choe GY, Park JK, Lisa JS, et al (2002) Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res 8:2894–2901
Presto MS (1999) Epidemiology. In: Berger MS, Wilson CB (eds) The gliomas. W.B. Saunders Co., Philadelphia, pp 2–11
Yoshida D, Watanabe K, Noha M, et al (2002) Suppression of matrix metalloproteinase activity by SI-27: detection by a new activity assay with s-24444, a specific chromogenic peptide. J Neurooncol 58:1–11
Yoshida D, Watanabe K, Noha M, et al (2003) Anti-invasive effect of an anti-matrix metalloproteinase agent in a murine brain slice model using the serial monitoring of green fluorescent protein-labeled glioma cells. Neurosurgery 52:187–197
Kachra Z, Beaulieu E, Delvecchi L, et al (1999) Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis 17:555–566
Lampert K, Machein U, Machein MR, et al (1998) Expression of matrix metalloproteinases and their tissure inhibitors in human brain tumor. Am J Pathol 153:429–437
Yamamoto M, Mohanam S, Sawaya R, et al (1996) Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase. A activation in human malignant brain tumors in vivo and in vitro. Cancer Res 56:384–392
Kondraganti S, Mohanam S, Chintala SK, et al (2000) Selective suppression of matrix metalloproteinase-9 in glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res 60:6851–6855
Deryugina EI, Bourdon MA, Luo GX, et al (1997) Matrix metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci 110:2473–2482
Rooprai HK, Rucklidge GJ, Panou C, et al (2000) The effect of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Br J Cancer 82:52–55
Sawaya RE, Yamamoto M, Gokaslan ZL, et al (1996) Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 14:35–42
Forsyth PA, Wong H, Liang TD, et al (1999) Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer Res 79:1828–1835
Cox G, Jones JL, O'Byne KJ, (2000) Matrix metalloproteinase 9 and the epidermal growth factor signal pathway in operable nonsmall cell lung cancer. Clin Cancer Res 6:2349–2355
Matrisian LM (1992) The matrix-degrading metalloproteinases. Bioessays 14:455–463
Giannelli G, Falk-Marzillier J, Schiraldi O, et al (1997) Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin-5. Science 277:225–228
Rao JS, Steck PA, Mohanam S, et al (1993) Elevated levels of M 9200 type IV collagenase in human brain tumor. Cancer Res 53:2208–2211
Reith A, Rucklidge GJ (1992) Invasion of brain tissue by primary glioma: evidence for the involvement of urokinase-type plasminogen activator as an activator of type IV collagenase. Biochem Biophys Res Commun 186:348–354
Yasuo S, Hiroyuki S, Robert CS, et al (1998) Matrix metalloproteinases-2 and-9 are expressed in human neuroblastoma: contribution to their production and correlation with metastasis. Cancer Res 58:2209–2216
Sallinen P, Hapasalo H, Kerttula T, et al (1994) Sources of variation in the assessment of cell proliferation using proliferating cell nuclear antigen immunohistochemistry. Anak Qyant Cytik Histol 16:261–268
Nakagawa T, Kuboto T, Kubota M (1996) Secretion of matrix metalloproteinase-2 (72 KD gelatinase/type IV collagnase-gelatinase A) by malignant human glioma cell lines: implication for the growth and cellular invasion of the extracellular matrix. J Neurooncol 28:16–24
Bergers G, Brekken R, McMahon G, et al (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2:737–744
Deryugina EI, Lao GX, Reisteld RA, et al (1997) Tumor cell invasion through matrigel is regulated by activated matrix metalloproteinases-2. Anticancer Res 17:3201–3210
Lamperl K, Maechein MR, Conea W (1998) Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol 153:429–437
Wang M, Fudage K, Rhim JS, et al (1996) Cytokine regulation of the matrix metalloproteinases-1 promoter via functional interaction of AP-1 and Ets-1 transcription factors. J Oncol Res 8:303–315
Raymond S, Yoshinori G, Athanassios PK, et al (1998) Elevated levels of Mr 92,000 type IV, collagenase during tumor growth in vivo. Biochem Biophys Res 251:632–636
Esteve PO, Tremblay M, St-Pierre R (1998) In vitro expression of MMP-2 and MMP-9 in glioma cells following exposure to inflammatory mediators. Biochem Biophys Acta 1403:885–896
Nakada M, Nakamura H, Ikeda E, et al (1991) Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol 154:417–428
Rooprai HK, Van Meter T, Rucklidge GJ, et al (1998) Comparative analysis of matrix metalloproteinases by immunocytochemistry, immunohistochemistry and zymography in human primary brain tumors. Int J Oncol 13:1153–1157
Wild-Bode C, Weller M, Wick W (2001) Molecular determinants of glioma cell migration and invasion. J Neurosurg 94:978–984
Lakka SS, Jasti SL, Kyritsis AP, et al (2000) Regulation of MMP-9 (type IV collagenase) production and invasiveness in gliomas by the extracellular signal-regulated kinase and jun amino-terminal kinase signaling cascades. Clin Exp Metastasis 18:245–252
Vince GH, Wagner S, Pietsch T, et al (1999) Heterogeneous regional expression patterns of matrix metalloproteinases in human malignant gliomas. Int J Dev Neurosci 17:437–445
Zucker S, Mirza H, Conner C, et al (1998) Vascular endothelial growth factor induces factor and matrix metalloproteinase in endothelial dothelial cell: conversion of prothrombin to thrombin results in progelatinase A activation and cell proliferation. Int J Cancer 75:780–786
Yoshida D, Noha M, Watanabe K, et al (2001) Novel approach to analysis of in vitro tumor angiogenesis with a variable-pressure scanning electron microscope: suppression by matrix metalloproteinase inhibitor SI-27. Brain Tumor Pathol 18:89–100
Lund E, Spang-Thomsen M, Skovgard-Poulsen H (1998) Tumor angiogenesis—a new therapeutic target in gliomas. Acta Neurol Scand 97:52–62
Muller D, Breathnach R, Engelmann A (1991) Expression of collagenase-related metalloproteinase genes in human lung of head and neck tumors. Int J Cancer 48:550–556
Muller D, Wolf C, Abecassis J, et al (1993) Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res 53:165–169
Venstrom KA, Reichardt LE (1993) Extracellular matrix: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 7:996–1003
Mohanam S, Wang WW, Rayford A, et al (1995) Expression of tissue inhibitors of metalloproteinases: negative regulators of human glioblastoma invasion in vivo. Clin Exp Metastasis 13:57–62
Noha M, Yoshida D, Watanabe K, et al (2000) Suppression of cell invasion on human malignant glioma cell lines by a novel matrix metalloproteinase inhibitor SI-27: in vitro study. J Neurooncol 48:217–223
Watanabe K, Yoshida D, Noha M, et al (2001) Suppression of matrix metalloproteinase-2 and-9 mediated invasiveness by a novel matrix metalloproteinase inhibitor, BE16627B. J Neurooncol 52:1–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Wang, T., Liu, S. et al. The expression of matrix metalloproteinase-2 and-9 in human gliomas of different pathological grades. Brain Tumor Pathol 20, 65–72 (2003). https://doi.org/10.1007/BF02483449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02483449