Abstract
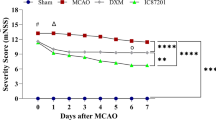
One approach for protecting neurons from excitotoxic damage in stroke is to attenuate receptor activity with specific antagonists. S-Methyl-N, N-diethylthiocarbamate sulfoxide (DETC-MeSO), the active metabolite of disulfiram, has been shown to be a partial antagonist of glutamate receptors and effective in reducing seizure. First, we investigated neuroprotective effect of DETC-MeSO on primary cortical neuronal culture under hypoxia/reoxygenation condition in vitro. Then, DETC-MeSO was administered subcutaneously for 4 and 8 days with the first injection occurring 1 h before or 24 h after reperfusion in the rat middle cerebral artery occlusion stroke model. Rats were subjected to the neuroscore test, and the brain was analyzed for infarct size. Monitoring neurotransmitter release was carried out by microdialysis. Heat shock proteins, key proteins involved in apoptosis and endoplasmic reticulum (ER) stress, were analyzed by immunoblotting. DETC-MeSO greatly reduced both cell death following hypoxia/reoxygenation and brain infarct size. It improved performance on the neuroscore test and attenuated proteolysis of αII-spectrin. The level of pro-apoptotic proteins declined, and anti-apoptotic and HSP27 protein expressions were markedly increased. Levels of the ER stress protein markers p-PERK, p-eIF2α, ATF4, JNK, XBP-1, GADD34, and CHOP significantly declined after DETC-MeSO administration. Microdialysis data showed that DETC-MeSO increased high potassium-induced striatal dopamine release indicating that more neurons were protected and survived under ischemic insult in the presence of DETC-MeSO. We also showed that DETC-MeSO can prevent gliosis. DETC-MeSO elicits neuroprotection through the preservation of ER resulting in reduction of apoptosis by increase of anti-apoptotic proteins and decrease of pro-apoptotic proteins.









Similar content being viewed by others
References
Nagendra SN, Faiman MD, Davis K, Wu JY, Newby X, Schloss JV (1997) Carbamoylation of brain glutamate receptors by a disulfiram metabolite. J Biol Chem 272:24247–24251
Ningaraj NS, Chen W, Schloss JV, Faiman MD, Wu J-Y (2001) S-Methyl-N, N-diethylthiocarbamate sulfoxide elicits neuroprotective effect against N-methyl-D-aspartate receptor-mediated neurotoxicity. J Biomed Sci 8:104–113
Hart BW, Faiman MD (1994) In vivo pharmacodynamic studies of the disulfiram metabolite S-methyl N, N-diethylthiolcarbamate sulfoxide: inhibition of liver aldehyde dehydrogenase. Alcohol Clin Exp Res 18:340–345
Broughton BRS, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339
Ma Y, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28:51–65
DeGracia DJ, Montie HL (2004) Cerebral ischemia and the unfolded protein response. J Neurochem 91:1–8
Gharibani PM, Modi J, Pan C, Menzie J, Ma Z, Chen P-C et al (2013) The mechanism of taurine protection against endoplasmic reticulum stress in an animal stroke model of cerebral artery occlusion and stroke-related conditions in primary neuronal cell culture. Adv Exp Med Biol 776:241–258
Li F, Omae T, Fisher M, Dietrich WD, Kuluz JW (1999) Spontaneous hyperthermia and its mechanism in the intraluminal suture middle cerebral artery occlusion model of rats. Editorial comment. Stroke 30:2464–2471
Menzies SA, Hoff JT, Betz AL (1992) Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery 31:100–106
Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C et al (2010) TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J Neurosci Methods 187:84–89
Mohammad-Gharibani P, Tiraihi T, Mesbah-Namin SA, Jalil Arabkheradmand HK (2012) Induction of bone marrow stromal cells into GABAergic neuronal phenotype using creatine as inducer. Restor Neurol Neurosci 30:511–525
Wu J, Matsuda T (1973) Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem 248:3029–3034
Solaroglu I, Tsubokawa T, Cahill J, Zhang JH (2006) Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience 143:965–974
Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M et al (2005) Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49:407–417
Savitz SI, Fisher M (2007) Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol 61:396–402
Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ (1998) A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke 29:2162–2170
Gerriets T, Stolz E, Walberer M, Müller C, Rottger C, Kluge A et al (2004) Complications and pitfalls in rat stroke models for middle cerebral artery occlusion: a comparison between the suture and the macrosphere model using magnetic resonance angiography. Stroke 35:2372–2377
Madan A, Parkinson A, Faiman MD (1995) Identification of the human and rat P450 enzymes responsible for the sulfoxidation of S-methyl N, N-diethylthiolcarbamate (DETC-ME). The terminal step in the bioactivation of disulfiram. Drug Metab Dispos 23:1153–1162
Schäbitz WR, Hoffmann TT, Heiland S, Kollmar R, Bardutzky J, Sommer C et al (2001) Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke 32:1226–1233
Waxman EA, Lynch DR (2005) N-Methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist 11:37–49
Pike BR, Flint J, Dutta S, Johnson E, Wang KK, Hayes RL (2001) Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J Neurochem 78:1297–1306
Wang KK, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian RV et al (1998) Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J Biol Chem 273:22490–22497
Zhang C, Siman R, Xu YA, Mills AM, Frederick JR, Neumar RW (2002) Comparison of calpain and caspase activities in the adult rat brain after transient forebrain ischemia. Neurobiol Dis 10:289–305
Pike BR, Zhao X, Newcomb JK, Wang KK, Posmantur RM, Hayes RL (1998) Temporal relationships between de novo protein synthesis, calpain and caspase 3-like protease activation, and DNA fragmentation during apoptosis in septo-hippocampal cultures. J Neurosci Res 52:505–520
Zhao X, Pike BR, Newcomb JK, Wang KK, Posmantur RM, Hayes RL (1999) Maitotoxin induces calpain but not caspase-3 activation and necrotic cell death in primary septo-hippocampal cultures. Neurochem Res 24:371–382
Nakka VP, Gusain A, Mehta SL, Raghubir R (2008) Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol 37:7–38
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Badin RA, Lythgoe MF, van der Weerd L, Thomas DL, Gadian DG, Latchman DS (2006) Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb Blood Flow Metab 26:371–381
Yenari MA (2002) Heat shock proteins and neuroprotection. Adv in Exp Med and Biol 513:281–299
Zourlidou A, Payne Smith MD, Latchman DS (2004) HSP27 but not HSP70 has a potent protective effect against alpha-synuclein-induced cell death in mammalian neuronal cells. J Neurochem 88:1439–1448
Whitlock NA, Lindsey K, Agarwal N, Crosson CE, Ma J-X (2005) Heat shock protein 27 delays Ca2+-induced cell death in a caspase-dependent and -independent manner in rat retinal ganglion cells. Invest Ophthalmol Vis Sci 46:1085–1091
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C et al (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652
Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE et al (1998) Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. J Neurosci 18:5891–5900
Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM (2005) Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19:2026–2028
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM et al (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661–665
Pap M, Cooper GM (1998) Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem 273:19929–19932
Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS (2003) Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem 278:20915–20924
Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD (2000) Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 23:222–229
Ni M, Zhang Y, Lee AS (2011) Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. J Biochem 434:181–188
Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y (2003) Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience 118:491–499
Kumar R, Azam S, Sullivan JM, Owen C, Cavener DR, Zhang P et al (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2alpha kinase, PERK. J Neurochem 77:1418–1421
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Chen X, Shen J, Prywes R (2002) The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277:13045–13052
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891
Lee A-H, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B et al (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949
Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C et al (1997) Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J Biol Chem 272:25238–25242
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 22:193–201
Mohammad-Gharibani P, Tiraihi T, Delshad A, Arabkheradmand J, Taheri T (2013) Improvement of contusive spinal cord injury in rats by co-transplantation of gamma-aminobutyric acid-ergic cells and bone marrow stromal cells. Cytother 15:1073–1085
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647
Wang C-C, Chio C-C, Chang C-H, Kuo J-R, Chang C-P (2010) Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol 10:11
Satoh JI, Kim SU (1995) Differential expression of heat shock protein HSP27 in human neurons and glial cells in culture. J Neurosci Res 41:805–818
Acknowledgments
This research was supported in part by grant 09KW-11, Department of Health, State of Florida.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad-Gharibani, P., Modi, J., Menzie, J. et al. Mode of Action of S-Methyl-N, N-Diethylthiocarbamate Sulfoxide (DETC-MeSO) as a Novel Therapy for Stroke in a Rat Model. Mol Neurobiol 50, 655–672 (2014). https://doi.org/10.1007/s12035-014-8658-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8658-0