Abstract
Stroke is the second most common cause of death and the leading cause of disability worldwide. Brain injury following stroke results from a complex series of pathophysiological events including excitotoxicity, oxidative and nitrative stress, inflammation, and apoptosis. Moreover, there is a mechanistic link between brain ischemia, innate and adaptive immune cells, intracranial atherosclerosis, and also the gut microbiota in modifying the cerebral responses to ischemic insult. There are very few treatments for stroke injuries, partly owing to an incomplete understanding of the diverse cellular and molecular changes that occur following ischemic stroke and that are responsible for neuronal death. Experimental discoveries have begun to define the cellular and molecular mechanisms involved in stroke injury, leading to the development of numerous agents that target various injury pathways. In the present article, we review the underlying pathophysiology of ischemic stroke and reveal the intertwined pathways that are promising therapeutic targets.


Similar content being viewed by others
References
Hossmann K-A (2006) Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol 26(7–8):1055–1081
Heron M (2007) Deaths: leading causes for 2004. Natl Vital Stat Rep 56(5):1–96
Tsuchiya M, Sako K, Yura S et al (1992) Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res 89(1):87–92
Seto S-W, Chang D, Jenkins A et al (2016) Angiogenesis in ischemic stroke and angiogenic effects of Chinese herbal medicine. Journal of clinical medicine 5(6):56
Fonarow GC, Zhao X, Smith EE et al (2014) Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 311(16):1632–1640
Del Zoppo GJ, Saver JL, Jauch EC et al (2009) Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the American Heart Association/American Stroke Association. Stroke 40(8):2945–2948
Sandercock P, Wardlaw JM, Lindley RI et al (2012) The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet (London, England) 379(9834):2352–2363
Amarenco P, Bogousslavsky J, Caplan L et al (2009) Classification of stroke subtypes. Cerebrovasc Dis 27(5):493–501
Beal CC (2010) Gender and stroke symptoms: a review of the current literature. J Neurosci Nurs 42(2):80–87
Hatano S (1976) Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 54(5):541
Murphy TH, Li P, Betts K et al (2008) Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci 28(7):1756–1772
Besancon E, Guo S, Lok J et al (2008) Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci 29(5):268–275
Bretón RR, Rodríguez JCG (2012) Excitotoxicity and oxidative stress in acute ischemic stroke. Stroke 8:9
Ouyang Y-B, Voloboueva LA, Xu L-J et al (2007) Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci 27(16):4253–4260
Xu L, Emery JF, Ouyang YB et al (2010) Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58(9):1042–1049
Siesjö B (1992) Pathophysiology and treatment of focal cerebral ischemia. II: Mechanisms of damage and treatment. J Neurosurg 77(3):337–354
Bandera E, Botteri M, Minelli C et al (2006) Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke a systematic review. Stroke 37(5):1334–1339
Baron J-C (1999) Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis 9(4):193–201
Jung S, Gilgen M, Slotboom J et al (2013) Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 136(Pt 12):3554–3560 awt246
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198
Edvinsson L, Krause DN (2002) Cerebral blood flow and metabolism. Eur J Neurol 9(5):550–550
Caplan L (2000) Caplan’s stroke: a clinical approach, 3rd edn. Butterworth Heinemann, Boston
Doyle KP, Simon RP, Stenzel-Poore MP (2008) Mechanisms of ischemic brain damage. Neuropharmacology 55(3):310–318
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188
Krnjević K (2008) Electrophysiology of cerebral ischemia. Neuropharmacology 55(3):319–333
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66
Olney JW, Price MT, Samson L et al (1986) The role of specific ions in glutamate neurotoxicity. Neurosci Lett 65(1):65–71
Rothman SM (1985) The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci 5(6):1483–1489
Choi DW (1985) Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 58(3):293–297
Tymianski M, Charlton MP, Carlen PL et al (1993) Secondary Ca 2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res 607(1):319–323
Pizzi M, Fallacara C, Arrighi V et al (1993) Attenuation of excitatory amino acid toxicity by metabotropic glutamate receptor agonists and aniracetam in primary cultures of cerebellar granule cells. J Neurochem 61(2):683–689
Mosbacher J, Schöpfer R, Monyer H et al (1994) A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266(5187):1059–1062
Moriyoshi K, Masu M, Ishii T et al (1991) Molecular cloning and characterization of the rat NMD receptor. Nature 354:31–37
Berdichevsky E, Riveros N, Sánchez-Armáss S et al (1983) Kainate, N-methylaspartate and other excitatory amino acids increase calcium influx into rat brain cortex cells in vitro. Neurosci Lett 36(1):75–80
Liu B, Liao M, Mielke JG et al (2006) Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci 26(20):5309–5319
Peng PL, Zhong X, Tu W et al (2006) ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron 49(5):719–733
Hsu CY (1998) Ischemic stroke: from basic mechanisms to new drug development, vol 16. Karger Medical and Scientific Publishers, Basel
Boscia F, Gala R, Pignataro G et al (2006) Permanent focal brain ischemia induces isoform-dependent changes in the pattern of Na+/Ca2+ exchanger gene expression in the ischemic core, periinfarct area, and intact brain regions. J Cereb Blood Flow Metab 26(4):502–517
Molinaro P, Cantile M, Cuomo O et al (2013) Neurounina-1, a novel compound that increases Na+/Ca2+ exchanger activity, effectively protects against stroke damage. Mol Pharmacol 83(1):142–156
Bano D, Young KW, Guerin CJ et al (2005) Cleavage of the plasma membrane Na+/Ca 2+ exchanger in excitotoxicity. Cell 120(2):275–285
Castilho RF, Hansson O, Ward MW et al (1998) Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 18(24):10277–10286
Abramov AY, Duchen MR (2008) Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1777(7):953–964
Ward MW, Rego AC, Frenguelli BG et al (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 20(19):7208–7219
Stout AK, Raphael HM, Kanterewicz BI et al (1998) Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci 1(5):366–373
White RJ, Reynolds IJ (1996) Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci 16(18):5688–5697
White RJ, Reynolds IJ (1997) Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol 498(Pt 1):31
Tymianski M, Charlton MP, Carlen PL et al (1993) Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci 13(5):2085–2104
Baudry M, Greget R, Pernot F et al (2012) Roles of group I metabotropic glutamate receptors under physiological conditions and in neurodegeneration. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1(4):523–532
Rong R, Ahn J-Y, Huang H et al (2003) PI3 kinase enhancer–Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci 6(11):1153–1161
Chong ZZ, Li F, Maiese K (2006) Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and β-catenin during oxidative stress. Curr Neurovasc Res 3(2):107–117
Hou L, Klann E (2004) Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24(28):6352–6361
Bruno V, Battaglia G, Copani A et al (2001) Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab 21(9):1013–1033
Yang Z-B, Zhang Z, Li T-B et al (2014) Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin Sci 127(12):679–689
Fang Q, Hu W-W, Wang X-F et al (2014) Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology 77:156–166
Lee J-M, Zipfel GJ, Choi DW (1999) The changing landscape of ischaemic brain injury mechanisms. Nature 399:A7–A14
Rothman SM, Olney JW (1995) Excitotoxicity and the NMDA receptor-still lethal after eight years. Trends Neurosci 18(2):57–58
Traynelis SF, Wollmuth LP, McBain CJ et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496
Collingridge GL, Peineau S, Howland JG et al (2010) Long-term depression in the CNS. Nat Rev Neurosci 11(7):459–473
Liu Y, Wong TP, Aarts M et al (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27(11):2846–2857
Chen M, Lu T-J, Chen X-J et al (2008) Differential roles of NMDA receptor subtypes in ischemic neuronal cell death and ischemic tolerance. Stroke 39(11):3042–3048
Ryan TJ, Emes RD, Grant SG et al (2008) Evolution of NMDA receptor cytoplasmic interaction domains: implications for organisation of synaptic signalling complexes. BMC Neurosci 9(1):6
Zhou M, Baudry M (2006) Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J Neurosci 26(11):2956–2963
DeRidder MN, Simon MJ, Siman R et al (2006) Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis 22(1):165–176
Terasaki Y, Sasaki T, Yagita Y et al (2010) Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J Cereb Blood Flow Metab 30(8):1441–1449
Harraz MM, Eacker SM, Wang X et al (2012) MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci 109(46):18962–18967
Martin HG, Wang YT (2010) Blocking the deadly effects of the NMDA receptor in stroke. Cell 140(2):174–176
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5(5):405–414
Tu W, Xu X, Peng L et al (2010) DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140(2):222–234
Zhou L, Li F, Xu H-B et al (2010) Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med 16(12):1439–1443
Lai TW, Shyu W-C, Wang YT (2011) Stroke intervention pathways: NMDA receptors and beyond. Trends Mol Med 17(5):266–275
Aluclu MU, Arslan S, Acar A et al (2008) Evaluation of effects of memantine on cerebral ischemia in rats. Neurosciences (Riyadh) 13(2):113–116
Okamoto S-I, Pouladi MA, Talantova M et al (2009) Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med 15(12):1407–1413
Aarts M, Liu Y, Liu L et al (2002) Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298(5594):846–850
Lai TW, Wang YT (2010) Fashioning drugs for stroke. Nat Med 16(12):1376–1378
Petralia RS, Wang Y-X, Hua F et al (2010) Organization of NMDA receptors at extrasynaptic locations. Neuroscience 167(1):68–87
Koumura A, Nonaka Y, Hyakkoku K et al (2008) A novel calpain inhibitor,((1S)-1 ((((1S)-1-benzyl-3-cyclopropylamino-2, 3-di-oxopropyl) amino) carbonyl)-3-methylbutyl) carbamic acid 5-methoxy-3-oxapentyl ester, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience 157(2):309–318
López-Menéndez C, Gascón S, Sobrado M et al (2009) Kidins220/ARMS downregulation by excitotoxic activation of NMDARs reveals its involvement in neuronal survival and death pathways. J Cell Sci 122(19):3554–3565
Xu J, Kurup P, Zhang Y et al (2009) Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci 29(29):9330–9343
Taghibiglou C, Martin HG, Lai TW et al (2009) Role of NMDA receptor—dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat Med 15(12):1399–1406
Beckman KB, Ames BN (1998) Mitochondrial aging: open questions. Ann N Y Acad Sci 854(1):118–127
Suh SW, Shin BS, Ma H et al (2008) Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol 64(6):654–663
Allen C, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4(6):461–470
Love S (1999) Oxidative stress in brain ischemia. Brain Pathol 9(1):119–131
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21(1):2–14
Kontos HA (2001) Oxygen radicals in cerebral ischemia the 2001 Willis lecture. Stroke 32(11):2712–2716
Cherubini A, Ruggiero C, Polidori MC et al (2005) Potential markers of oxidative stress in stroke. Free Radic Biol Med 39(7):841–852
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262(5134):689–695
Cuzzocrea S, Riley DP, Caputi AP et al (2001) Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev 53(1):135–159
Lafon-Cazal M, Pietri S, Culcasi M et al (1993) NMDA-dependent superoxide production and neurotoxicity. Nature 364(6437):535–537
Piantadosi CA, Zhang J (1996) Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke 27(2):327–332
Sugawara T, Chan PH (2003) Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 5(5):597–607
Saeed SA, Shad KF, Saleem T et al (2007) Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp Brain Res 182(1):1–10
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12(1):125–169
Girouard H, Wang G, Gallo EF et al (2009) NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29(8):2545–2552
Brennan AM, Suh SW, Won SJ et al (2009) NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12(7):857–863
Nicholls DG (2008) Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci 1147(1):53–60
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27(5):1129–1138
Förstermann U (2010) Nitric oxide and oxidative stress in vascular disease. Pflügers Archiv-European Journal of Physiology 459(6):923–939
Wei EP, Kontos HA, Beckman JS (1996) Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Phys Heart Circ Phys 271(3):H1262–H1266
Aarts MM, Tymianski M (2005) TRPMs and neuronal cell death. Pflugers Arch 451(1):243–249
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87(1):315–424
Nakamura T, Lipton SA (2009) According to GOSPEL: filling in the GAP (DH) of NO-mediated neurotoxicity. Neuron 63(1):3–6
Gu Z, Kaul M, Yan B et al (2002) S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science 297(5584):1186–1190
Faraci FM (2006) Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol 100(2):739–743
Lipton SA (2007) Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci 8(10):803–808
Neumar RW (2000) Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med 36(5):483–506
Gariballa S, Hutchin T, Sinclair A (2002) Antioxidant capacity after acute ischaemic stroke. QJM 95(10):685–690
Spranger M, Krempien S, Schwab S et al (1997) Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury correlation with clinical course and infarct size. Stroke 28(12):2425–2428
Alfieri A, Srivastava S, Siow R et al (2011) Targeting the Nrf2–Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol 589(17):4125–4136
Margaill I, Plotkine M, Lerouet D (2005) Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39(4):429–443
Zhang C, Shu L, Kong A-N T (2015) MicroRNAs: new players in cancer prevention targeting Nrf2, oxidative stress and inflammatory pathways. Current pharmacology reports 1(1):21–30
Johnson JA, Johnson DA, Kraft AD et al (2008) The Nrf2–ARE pathway. Ann N Y Acad Sci 1147(1):61–69
Hardingham GE, Lipton SA (2011) Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid Redox Signal 14(8):1421–1424
Dang J, Brandenburg L-O, Rosen C et al (2012) Nrf2 expression by neurons, astroglia, and microglia in the cerebral cortical penumbra of ischemic rats. J Mol Neurosci 46(3):578–584
Joshi GA, Johnson J (2012) The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent patents on CNS drug discovery 7(3):218–229
Jiang S, Deng C, Lv J et al (2016) Nrf2 weaves an elaborate network of neuroprotection against stroke. Mol Neurobiol 1–16
Zhang M, An C, Gao Y et al (2013) Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol 100:30–47
Liou AK, Clark RS, Henshall DC et al (2003) To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol 69(2):103–142
Chamorro Á, Hallenbeck J (2006) The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 37(2):291–293
McColl B, Allan S, Rothwell N (2009) Systemic infection, inflammation and acute ischemic stroke. Neuroscience 158(3):1049–1061
Pan J, Palmateer J, Schallert T et al (2014) Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke. Translational stroke research 5(5):577–585
Jeong H-K, Ji K, Min K et al (2013) Brain inflammation and microglia: facts and misconceptions. Exp Neurobiol 22(2):59–67
Amantea D, Nappi G, Bernardi G et al (2009) Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J 276(1):13–26
Kriz J (2006) Inflammation in ischemic brain injury: timing is important. Critical reviews™ in Neurobiology 18 (1–2):
Stanimirovic DB, Wong J, Shapiro A et al (1997) Increase in surface expression of ICAM-1, VCAM-1 and E-selectin in human cerebromicrovascular endothelial cells subjected to ischemia-like insults. In: Brain Edema X. Springer, Berlin Heidelberg New York, p 12–16
Becker K (1998) Inflammation and acute stroke. Curr Opin Neurol 11(1):45–49
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7(1):1
Gan Y, Liu Q, Wu W et al (2014) Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci 111(7):2704–2709
Rogove A, Lu W, Tsirka S (2002) Microglial activation and recruitment, but not proliferation, suffice to mediate neurodegeneration. Cell Death Differ 9(8):801–806
Graeber MB, Streit WJ (2010) Microglia: biology and pathology. Acta Neuropathol 119(1):89–105
McKimmie CS, Roy D, Forster T et al (2006) Innate immune response gene expression profiles of N9 microglia are pathogen-type specific. J Neuroimmunol 175(1):128–141
Hoehn BD, Palmer TD, Steinberg GK (2005) Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke 36(12):2718–2724
Pena-Philippides JC, Yang Y, Bragina O et al (2014) Effect of pulsed electromagnetic field (PEMF) on infarct size and inflammation after cerebral ischemia in mice. Translational stroke research 5(4):491–500
Yilmaz G, Granger DN (2008) Cell adhesion molecules and ischemic stroke. Neurol Res 30(8):783–793
Mrak RE, Griffin WST (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 26(3):349–354
Eikelenboom P, Rozemuller AJ, Hoozemans JJ et al (2000) Neuroinflammation and Alzheimer disease: clinical and therapeutic implications. Alzheimer Dis Assoc Disord 14(1):S54–S61
Lucas SM, Rothwell NJ, Gibson RM (2006) The role of inflammation in CNS injury and disease. Br J Pharmacol 147(S1):S232–S240
Fernandes A, Miller-Fleming L, Pais TF (2014) Microglia and inflammation: conspiracy, controversy or control? Cell Mol Life Sci 71(20):3969–3985
Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140(6):871–882
Widgerow AD (2012) Cellular resolution of inflammation—catabasis. Wound Repair Regen 20(1):2–7
Fleming JC, Norenberg MD, Ramsay DA et al (2006) The cellular inflammatory response in human spinal cords after injury. Brain 129(12):3249–3269
Ross AM, Hurn P, Perrin N et al (2007) Evidence of the peripheral inflammatory response in patients with transient ischemic attack. J Stroke Cerebrovasc Dis 16(5):203–207
Greenwood J, Heasman S, Alvarez J et al (2011) Review: leucocyte–endothelial cell crosstalk at the blood–brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol 37(1):24–39
Yang MS, Min KJ, Joe E (2007) Multiple mechanisms that prevent excessive brain inflammation. J Neurosci Res 85(11):2298–2305
Vincent V, Tilders F, Van Dam AM (1997) Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor β. Glia 19(3):190–198
Pyo H, Yang M-S, Jou I et al (2003) Wortmannin enhances lipopolysaccharide-induced inducible nitric oxide synthase expression in microglia in the presence of astrocytes in rats. Neurosci Lett 346(3):141–144
Min K-J, Yang M-S, Kim S-U et al (2006) Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci 26(6):1880–1887
Kim JH, Min KJ, Seol W et al (2010) Astrocytes in injury states rapidly produce anti-inflammatory factors and attenuate microglial inflammatory responses. J Neurochem 115(5):1161–1171
Kim B, Jeong H-K, Kim J-H et al (2011) Uridine 5′-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol 186(6):3701–3709
Hoek RM, Ruuls SR, Murphy CA et al (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290(5497):1768–1771
Cardona AE, Pioro EP, Sasse ME et al (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924
Kim YS, Kim SS, Cho JJ et al (2005) Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci 25(14):3701–3711
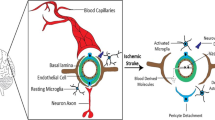
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808
Swanson RA, Ying W, Kauppinen TM (2004) Astrocyte influences on ischemic neuronal death. Curr Mol Med 4(2):193–205
Ferrarese C, Mascarucci P, Zoia C et al (1999) Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab 19(9):1004–1009
Fu Y, Liu Q, Anrather J et al (2015) Immune interventions in stroke. Nat Rev Neurol 11(9):524–535
Chamorro Á, Meisel A, Planas AM et al (2012) The immunology of acute stroke. Nat Rev Neurol 8(7):401–410
Gelderblom M, Leypoldt F, Steinbach K et al (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40(5):1849–1857
Hammond MD, Taylor RA, Mullen MT et al (2014) CCR2+ Ly6Chi inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci 34(11):3901–3909
Zhang R, Chopp M, Zhang Z et al (1998) The expression of P-and E-selectins in three models of middle cerebral artery occlusion. Brain Res 785(2):207–214
Huang J, Upadhyay UM, Tamargo RJ (2006) Inflammation in stroke and focal cerebral ischemia. Surg Neurol 66(3):232–245
Chou WH, Choi DS, Zhang H et al (2004) Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J Clin Invest 114(1):49–56
Liu T, Clark R, McDonnell P et al (1994) Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 25(7):1481–1488
Zhu Y, Yang G-Y, Ahlemeyer B et al (2002) Transforming growth factor-β1 increases bad phosphorylation and protects neurons against damage. J Neurosci 22(10):3898–3909
Spera PA, Ellison JA, Feuerstein GZ et al (1998) IL-10 reduces rat brain injury following focal stroke. Neurosci Lett 251(3):189–192
Vila N, Castillo J, Dávalos A et al (2003) Levels of anti-inflammatory cytokines and neurological worsening in acute ischemic stroke. Stroke 34(3):671–675
Viviani B, Bartesaghi S, Gardoni F et al (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23(25):8692–8700
Bernardes-Silva M, Anthony DC, Issekutz AC et al (2001) Recruitment of Neutrophils across the blood–brain barrier: the role of E-and P-selectins. J Cereb Blood Flow Metab 21(9):1115–1124
Konsman JP, Vigues S, Mackerlova L et al (2004) Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol 472(1):113–129
Mazzotta G, Sarchielli P, Caso V et al (2004) Different cytokine levels in thrombolysis patients as predictors for clinical outcome. Eur J Neurol 11(6):377–381
Bö L, Peterson JW, Mørk S et al (1996) Distribution of immunoglobulin superfamily members ICAM-1,-2,-3, and the β2 integrin LFA-1 in multiple sclerosis lesions. J Neuropathol Exp Neurol 55(10):1060–1072
Huang FP, Wang ZQ, Wu DC et al (2003) Early NFκB activation is inhibited during focal cerebral ischemia in interleukin-1β-converting enzyme deficient mice. J Neurosci Res 73(5):698–707
Ohtaki H, Takaki A, Yin L et al (2003) Suppression of oxidative stress after transient focal ischemia in interleukin-1 knock out mice. In: Brain Edema XII. Springer, Berlin Heidelberg New York, p 191–194
Wang X, Yue T-L, Young PR et al (1995) Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. J Cereb Blood Flow Metab 15(1):166–171
Tarkowski E, Rosengren L, Blomstrand C et al (1995) Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 26(8):1393–1398
Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8(9):1254–1266
Hakkoum D, Stoppini L, Muller D (2007) Interleukin-6 promotes sprouting and functional recovery in lesioned organotypic hippocampal slice cultures. J Neurochem 100(3):747–757
Tancredi V, D'Antuono M, Cafè C et al (2000) The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem 75(2):634–643
Relton J, Martin D, Thompson R et al (1996) Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol 138(2):206–213
Azzimondi G, Bassein L, Nonino F et al (1995) Fever in acute stroke worsens prognosis: a prospective study. Stroke 26(11):2040–2043
Zaremba J, Skrobanski P, Losy J (2001) Tumour necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct. Biomed Pharmacother 55(5):258–263
Dawson DA, Martin D, Hallenbeck JM (1996) Inhibition of tumor necrosis factor-alpha reduces focal cerebral ischemic injury in the spontaneously hypertensive rat. Neurosci Lett 218(1):41–44
Lavine SD, Hofman FM, Zlokovic BV (1998) Circulating antibody against tumor necrosis factor-alpha protects rat brain from reperfusion injury. J Cereb Blood Flow Metab 18(1):52–58
Nawashiro H, Martin D, Hallenbeck JM (1997) Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab 17(2):229–232
Nawashiro H, Martin D, Hallenbeck JM (1997) Neuroprotective effects of TNF binding protein in focal cerebral ischemia. Brain Res 778(2):265–271
Ooboshi H, Ibayashi S, Shichita T et al (2005) Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation 111(7):913–919
Liesz A, Suri-Payer E, Veltkamp C et al (2009) Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15(2):192–199
Iadecola C, Anrather J (2011) Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 14(11):1363–1368
McNeill H, Williams C, Guan J et al (1994) Neuronal rescue with transforming growth factor-[beta] 1 after hypoxic-ischaemic brain injury. Neuroreport 5(8):901–904
Mori E, Del Zoppo GJ, Chambers JD et al (1992) Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke 23(5):712–718
Nurmi A, Lindsberg PJ, Koistinaho M et al (2004) Nuclear factor-κB contributes to infarction after permanent focal ischemia. Stroke 35(4):987–991
Hayden MS, Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132(3):344–362
Ridder D, Schwaninger M (2009) NF-κB signaling in cerebral ischemia. Neuroscience 158(3):995–1006
Han H, Yenari M (2003) Cellular targets of brain inflammation in stroke. Current opinion in investigational drugs (London, England: 2000) 4(5):522–529
Baeuerle P, Henkel T (1994) Dunction and activation of NF-B in the immune system. Annu Rev Immunol 12(141):79
Ko HM, Koppula S, Kim B-W et al (2010) Inflexin attenuates proinflammatory responses and nuclear factor-ΚB activation in LPS-treated microglia. Eur J Pharmacol 633(1):98–106
Jin H, Zhu ZG, Yu PJ et al (2012) Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-κB signalling pathway activation. Phytother Res 26(9):1320–1326
Wang X, Hu D, Zhang L et al (2014) Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB/MAPKs pathway. Food Chem Toxicol 63:119–127
Montaner J, Alvarez-Sabín J, Molina C et al (2001) Matrix metalloproteinase expression after human cardioembolic stroke temporal profile and relation to neurological impairment. Stroke 32(8):1759–1766
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62(2):127–136
Jin R, Yang G, Li G (2010) Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 38(3):376–385
Asahi M, Wang X, Mori T et al (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci 21(19):7724–7732
Zhao B-Q, Wang S, Kim H-Y et al (2006) Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med 12(4):441–445
Weinstein JR, Koerner IP, Möller T (2010) Microglia in ischemic brain injury. Future Neurol 5(2):227–246
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11(5):373–384
Karikó K, Ni H, Capodici J et al (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279(13):12542–12550
Marsh BJ, Stenzel-Poore MP (2008) Toll-like receptors: novel pharmacological targets for the treatment of neurological diseases. Curr Opin Pharmacol 8(1):8–13
Brea D, Blanco M, Ramos-Cabrer P et al (2011) Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J Cereb Blood Flow Metab 31(6):1424–1431
Caso JR, Pradillo JM, Hurtado O et al (2007) Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115(12):1599–1608
Yao L, Kan EM, Lu J et al (2013) Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation 10(1):23
Hyakkoku K, Hamanaka J, Tsuruma K et al (2010) Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 171(1):258–267
Lehnardt S, Lehmann S, Kaul D et al (2007) Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol 190(1):28–33
Cao C-X, Yang Q-w, Lv F-L et al (2007) Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun 353(2):509–514
Bohacek I, Cordeau P, Lalancette-Hébert M et al (2012) Toll-like receptor 2 deficiency leads to delayed exacerbation of ischemic injury. J Neuroinflammation 9(1):1
Akira S (2006) TLR signaling, in from innate immunity to immunological memory. Springer, Berlin Heidelberg New York, pp 1–16
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8(4):279–289
Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ (2016) Inflammatory disequilibrium in stroke. Circ Res 119(1):142–158
Garcia-Bonilla L, Iadecola C (2012) Peroxiredoxin sets the brain on fire after stroke. Nat Med 18(6):858–859
Benakis C, Garcia-Bonilla L, Iadecola C et al (2015) The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci 8:461
Singh V, Roth S, Veltkamp R et al (2016) HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid Redox Signal 24(12):635–651
Marsh BJ, Williams-Karnesky RL, Stenzel-Poore MP (2009) Toll-like receptor signaling in endogenous neuroprotection and stroke. Neuroscience 158(3):1007–1020
Anrather J, Iadecola C (2016) Inflammation and stroke: an overview. Neurotherapeutics 1–10
Schilling M, Strecker J-K, Ringelstein EB et al (2009) The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res 1289:79–84
Konsman JP, Drukarch B, Van Dam A-M (2007) (Peri) vascular production and action of pro-inflammatory cytokines in brain pathology. Clin Sci 112(1):1–25
Mantovani A, Sica A, Locati M (2005) Macrophage polarization comes of age. Immunity 23(4):344–346
Connolly ES Jr, Winfree CJ, Springer TA et al (1996) Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 97(1):209
Connolly E, Winfree C, Prestigiacomo C et al (1997) Exacerbation of cerebral injury in mice that express the P-selectin gene. Circ Res 81(3):304–310
Soriano SG, Lipton SA, Wang YF et al (1996) Intercellular adhesion molecule-1-deficient mice are less susceptible to cerebral ischemia-reperfusion lnjury. Ann Neurol 39(5):618–624
Justicia C, Panés J, Solé S et al (2003) Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab 23(12):1430–1440
Ajmo CT, Collier LA, Leonardo CC et al (2009) Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol 218(1):47–55
Felger JC, Abe T, Kaunzner UW et al (2010) Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun 24(5):724–737
Kostulas N, Li H-L, Xiao B-G et al (2002) Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke 33(4):1129–1134
Yilmaz A, Fuchs T, Dietel B et al (2009) Transient decrease in circulating dendritic cell precursors after acute stroke: potential recruitment into the brain. Clin Sci 118(2):147–157
Saino O, Taguchi A, Nakagomi T et al (2010) Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke. J Neurosci Res 88(11):2385–2397
Santana M, Rosenstein Y (2003) What it takes to become an effector T cell: the process, the cells involved, and the mechanisms. J Cell Physiol 195(3):392–401
Collison LW, Workman CJ, Kuo TT et al (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569
Niedbala W, Wei XQ, Cai B et al (2007) IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37(11):3021–3029
Shichita T, Sugiyama Y, Ooboshi H et al (2009) Pivotal role of cerebral interleukin-17–producing γδT cells in the delayed phase of ischemic brain injury. Nat Med 15(8):946–950
Liesz A, Karcher S, Veltkamp R (2013) Spectratype analysis of clonal T cell expansion in murine experimental stroke. J Neuroimmunol 257(1):46–52
Ren X, Akiyoshi K, Dziennis S et al (2011) Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci 31(23):8556–8563
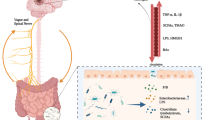
Benakis C, Brea D, Caballero S et al (2016) Commensal microbiota affects ischemic stroke outcome by regulating intestinal [gamma][delta] T cells. Nat Med 22:516–523
Singh V, Roth S, Llovera G et al (2016) Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci 36(28):7428–7440
Karlsson FH, Fåk F, Nookaew I et al (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3:1245
Yin J, Liao SX, He Y et al (2015) Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 4(11):e002699
Arpaia N, Campbell C, Fan X et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455
Zhou W, Liesz A, Bauer H et al (2013) Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models. Brain Pathol 23(1):34–44
Vandenabeele P, Galluzzi L, Berghe TV et al (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714
Wei L, Ying D-J, Cui L et al (2004) Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res 1022(1):54–61
Ünal-Çevik I, Kılınç M, Can A et al (2004) Apoptotic and necrotic death mechanisms are concomitantly activated in the same cell after cerebral ischemia. Stroke 35(9):2189–2194
Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17(20):2481–2495
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1):99–163
Nikoletopoulou V, Markaki M, Palikaras K et al (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1833(12):3448–3459
Culmsee C, Zhu C, Landshamer S et al (2005) Apoptosis-inducing factor triggered by poly (ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci 25(44):10262–10272
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40(5):e331–e339
Li H, Colbourne F, Sun P et al (2000) Caspase inhibitors reduce neuronal injury after focal but not global cerebral ischemia in rats. Stroke 31(1):176–182
Hickey EJ, You X, Kaimaktchiev V et al (2007) Lipopolysaccharide preconditioning induces robust protection against brain injury resulting from deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg 133(6):1588–1596
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6(5)
Adams JM, Cory S (2001) Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci 26(1):61–66
Antonsson B, Montessuit S, Sanchez B et al (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 276(15):11615–11623
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407(6805):770–776
Zoppo G, Ginis I, Hallenbeck JM et al (2000) Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 10(1):95–112
Zoppo GJ (1997) Microvascular responses to cerebral ischemia/inflammation. Ann N Y Acad Sci 823(1):132–147
Jin K, Graham SH, Mao X et al (2001) Fas (CD95) may mediate delayed cell death in hippocampal CA1 sector after global cerebral ischemia. J Cereb Blood Flow Metab 21(12):1411–1421
Namura S, Zhu J, Fink K et al (1998) Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18(10):3659–3668
Green DR (2005) Apoptotic pathways: ten minutes to dead. Cell 121(5):671–674
Ferrer I, Planas AM (2003) Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62(4):329–339
Seko Y, Kayagaki N, K-i S et al (2002) Role of Fas/FasL pathway in the activation of infiltrating cells in murine acute myocarditis caused by Coxsackievirus B3. J Am Coll Cardiol 39(8):1399–1403
Ma J, Endres M, Moskowitz MA (1998) Synergistic effects of caspase inhibitors and MK-801 in brain injury after transient focal cerebral ischaemia in mice. Br J Pharmacol 124(4):756–762
Graham SH, Chen J (2001) Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21(2):99–109
Wei N, Xiao L, Xue R et al (2015) MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol 1–9
Luo S, Rubinsztein DC (2013) BCL2L11/BIM: a novel molecular link between autophagy and apoptosis. Autophagy 9(1):104–105
Sionov RV, Vlahopoulos SA, Granot Z (2015) Regulation of Bim in health and disease. Oncotarget 6(27):23058
Yin K-J, Deng Z, Huang H et al (2010) miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis 38(1):17–26
Moon J-M, Xu L, Giffard RG (2013) Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab 33(12):1976–1982
Huang W, Liu X, Cao J et al (2015) miR-134 regulates ischemia/reperfusion injury-induced neuronal cell death by regulating CREB signaling. J Mol Neurosci 55(4):821–829
Shinoura N, Satou R, Yoshida Y et al (2000) Adenovirus-mediated transfer of Bcl-X L protects neuronal cells from Bax-induced apoptosis. Exp Cell Res 254(2):221–231
Zhao H, Yenari MA, Cheng D et al (2003) Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85(4):1026–1036
Gonzalez R, Hirsch J, Koroshetz W et al (2007) Acute ischemic stroke: imaging and intervention. Am J Neuroradiol 28(8):1622
Guan Q-H, Pei D-S, Liu X-M et al (2006) Neuroprotection against ischemic brain injury by SP600125 via suppressing the extrinsic and intrinsic pathways of apoptosis. Brain Res 1092(1):36–46
Guan Q-H, Pei D-S, Zong Y-Y et al (2006) Neuroprotection against ischemic brain injury by a small peptide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and non-nuclear pathways. Neuroscience 139(2):609–627
Kim JS, Kim Y-J, Ahn S-H et al (2016) Location of cerebral atherosclerosis: why is there a difference between East and West? Int J Stroke
Ritz K, Denswil NP, Stam OC et al (2014) Cause and mechanisms of intracranial atherosclerosis. Circulation 130(16):1407–1414
Suri MFK, Qiao Y, Ma X et al (2016) Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population. Stroke 47(5):1187–1193
Hu X, De Silva TM, Chen J et al (2017) Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ Res 120(3):449–471
Hollander W, Prusty S, Kemper T et al (1993) The effects of hypertension on cerebral atherosclerosis in the cynomolgus monkey. Stroke 24(8):1218–1226
Arvanitakis Z, Capuano AW, Leurgans SE et al (2016) Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. The Lancet Neurology 15(9):934–943
Roher AE, Esh C, Kokjohn TA et al (2003) Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol 23(11):2055–2062
Gupta A, Iadecola C (2015) Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci 16(7):115
Ballinger SW, Patterson C, Knight-Lozano CA et al (2002) Mitochondrial integrity and function in atherogenesis. Circulation 106(5):544–549
Napoli C, Witztum JL, de Nigris F et al (1999) Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation 99(15):2003–2010
D’armiento FP, Bianchi A, de Nigris F et al (2001) Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 32(11):2472–2480
Wang Z, Roberts AB, Buffa JA et al (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163(7):1585–1595
Kinlay S, Michel T, Leopold JA (2016) The future of vascular biology and medicine. Circulation 133(25):2603–2609
Zhu W, Gregory JC, Org E et al (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165(1):111–124
Shobha N, Buchan AM, Hill MD et al (2010) Thrombolysis at 3–4.5 hours after acute ischemic stroke onset–evidence from the Canadian Alteplase for Stroke Effectiveness Study (CASES) registry. Cerebrovasc Dis 31(3):223–228
Parmar S, Moore-Langston S, Fredrickson V et al (2015) Neuroprotective mechanisms of oxygen and ethanol: a potential combination therapy in stroke. Curr Med Chem 22(10):1194–1204
Geng X, Fu P, Ji X et al (2013) Synergetic neuroprotection of normobaric oxygenation and ethanol in ischemic stroke through improved oxidative mechanism. Stroke 44(5):1418–1425
Geng X, Parmar S, Li X et al (2013) Reduced apoptosis by combining normobaric oxygenation with ethanol in transient ischemic stroke. Brain Res 1531:17–24
Geng X, Sy CA, Kwiecien TD et al (2015) Reduced cerebral monocarboxylate transporters and lactate levels by ethanol and normobaric oxygen therapy in severe transient and permanent ischemic stroke. Brain Res 1603:65–75
Choi K-E, Hall CL, Sun J-M et al (2012) A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J 26(7):2799–2810
Katz LM, Young AS, Frank JE et al (2004) Regulated hypothermia reduces brain oxidative stress after hypoxic-ischemia. Brain Res 1017(1):85–91
Truettner JS, Suzuki T, Dietrich WD (2005) The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Mol Brain Res 138(2):124–134
Lee JH, Wei L, Gu X et al (2014) Therapeutic effects of pharmacologically induced hypothermia against traumatic brain injury in mice. J Neurotrauma 31(16):1417–1430
Polderman KH, Joe RTT, Peerdeman SM et al (2002) Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med 28(11):1563–1573
Lee JH, Wei ZZ, Cao W et al (2016) Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis 96:248–260
Zausinger S, Schöller K, Plesnila N et al (2003) Combination drug therapy and mild hypothermia after transient focal cerebral ischemia in rats. Stroke 34(9):2246–2251
Kollmar R, Henninger N, Bardutzky J et al (2004) Combination therapy of moderate hypothermia and thrombolysis in experimental thromboembolic stroke—an MRI study. Exp Neurol 190(1):204–212
Zhao H, Shimohata T, Wang JQ et al (2005) Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 25(42):9794–9806
Zhao H, Yenari MA, Sapolsky RM et al (2004) Mild postischemic hypothermia prolongs the time window for gene therapy by inhibiting cytochrome C release. Stroke 35(2):572–577
Faillace MP, Keller Sarmiento MI, Rosenstein RE (1996) Melatonin effect on [3H] glutamate uptake and release in the golden hamster retina. J Neurochem 67(2):623–628
Qian Y, Tang X, Guan T et al (2016) Neuroprotection by combined administration with maslinic acid, a natural product from Olea europaea, and MK-801 in the cerebral ischemia model. Molecules 21(8):1093
Cuccione E, Padovano G, Versace A et al (2016) Cerebral collateral circulation in experimental ischemic stroke. Exp Transl Stroke Med 8(1):2
Jackman K, Iadecola C (2015) Neurovascular regulation in the ischemic brain. Antioxid Redox Signal 22(2):149–160
Bang OY, Saver JL, Buck BH et al (2008) Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 79(6):625–629
Lima FO, Furie KL, Silva GS et al (2010) The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke 41(10):2316–2322
Shuaib A, Butcher K, Mohammad AA et al (2011) Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. The Lancet Neurology 10(10):909–921
Mergenthaler P, Dirnagl U (2011) Protective conditioning of the brain: expressway or roadblock? J Physiol 589(17):4147–4155
Hess DC, Hoda MN, Bhatia K (2013) Remote limb perconditioning and postconditioning. Stroke 44(4):1191–1197
Stevens SL, Vartanian KB, Stenzel-Poore MP (2014) Reprogramming the response to stroke by preconditioning. Stroke 45(8):2527–2531
Pignataro G, Scorziello A, Di Renzo G et al (2009) Post-ischemic brain damage: effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J 276(1):46–57
Zhao H, Joo S, Xie W et al (2013) Using hormetic strategies to improve ischemic preconditioning and postconditioning against stroke. Int J Physiol Pathophysiol Pharmacol 5(2):61–72
Meng R, Asmaro K, Meng L et al (2012) Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology 79(18):1853–1861
Stowe AM, Wacker BK, Cravens PD et al (2012) CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke. J Neuroinflammation 9(1):33
Ouk T, Laprais M, Bastide M et al (2009) Withdrawal of fenofibrate treatment partially abrogates preventive neuroprotection in stroke via loss of vascular protection. Vasc Pharmacol 51(5):323–330
Khan MB, Hoda MN, Vaibhav K et al (2015) Remote ischemic postconditioning: harnessing endogenous protection in a murine model of vascular cognitive impairment. Translational stroke research 6(1):69–77
Stowe AM, Altay T, Freie AB et al (2011) Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol 69(6):975–985
Bowen KK, Naylor M, Vemuganti R (2006) Prevention of inflammation is a mechanism of preconditioning-induced neuroprotection against focal cerebral ischemia. Neurochem Int 49(2):127–135
Joo SP, Xie W, Xiong X et al (2013) Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience 243:149–157
Dai Y, Li W, Zhong M et al (2014) Preconditioning and post-treatment with cobalt chloride in rat model of perinatal hypoxic–ischemic encephalopathy. Brain and Development 36(3):228–240
Pignataro G, Meller R, Inoue K et al (2008) In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab 28(2):232–241
Acknowledgements
This work was supported by Ahvaz Jundishapur University Grant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Khoshnam, S.E., Winlow, W., Farzaneh, M. et al. Pathogenic mechanisms following ischemic stroke. Neurol Sci 38, 1167–1186 (2017). https://doi.org/10.1007/s10072-017-2938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2938-1