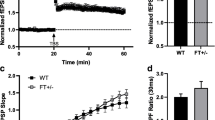
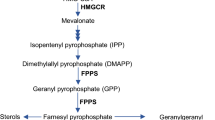
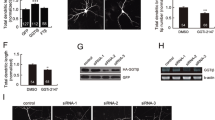
Abstract
Protein prenylation is an important lipid posttranslational modification of proteins. It includes protein farnesylation and geranylgeranylation, in which the 15-carbon farnesyl pyrophosphate or 20-carbon geranylgeranyl pyrophosphate is attached to the C-terminus of target proteins, catalyzed by farnesyl transferase or geranylgeranyl transferases, respectively. Protein prenylation facilitates the anchoring of proteins into the cell membrane and mediates protein–protein interactions. Among numerous proteins that undergo prenylation, small GTPases represent the largest group of prenylated proteins. Small GTPases are involved in regulating a plethora of cellular functions including synaptic plasticity. The prenylation status of small GTPases determines the subcellular locations and functions of the proteins. Dysregulation or dysfunction of small GTPases leads to the development of different types of disorders. Emerging evidence indicates that prenylated proteins, in particular small GTPases, may play important roles in the pathogenesis of Alzheimer’s disease. This review focuses on the prenylation of Ras and Rho subfamilies of small GTPases and its relation to synaptic plasticity and Alzheimer’s disease.



Similar content being viewed by others
References
Krishna RG, Wold F (1993) Post-translational modification of proteins. Adv Enzymol Relat Areas Mol Biol 67:265–298
Lane KT, Beese LS (2006) Thematic review series: lipid posttranslational modifications. Structural biology of protein farnesyltransferase and geranylgeranyltransferase type I. J Lipid Res 47(4):681–699
Alzheimer's Association (2013) 2013 Alzheimer's disease facts and figures. Alzheimers Dement 9(2):208–245. doi:10.1016/j.jalz.2013.02.003
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1(1):a006189. doi:10.1101/cshperspect.a006189
Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298(5594):789–791
Cole SL, Vassar R (2006) Isoprenoids and Alzheimer's disease: a complex relationship. Neurobiol Dis 22(2):209–222
Hooff GP, Wood WG, Muller WE, Eckert GP (2010) Isoprenoids, small GTPases and Alzheimer's disease. Biochim Biophys Acta 1801(8):896–905
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343(6257):425–430
Leung KF, Baron R, Seabra MC (2006) Thematic review series: lipid posttranslational modifications. geranylgeranylation of Rab GTPases. J Lipid Res 47(3):467–475
McTaggart SJ (2006) Isoprenylated proteins. Cell Mol Life Sci 63(3):255–267
Liao JK (2002) Isoprenoids as mediators of the biological effects of statins. J Clin Invest 110(3):285–288
Vaughan CJ (2003) Prevention of stroke and dementia with statins: effects beyond lipid lowering. Am J Cardiol 91(4A):23B–29B
Berndt N, Hamilton AD, Sebti SM (2011) Targeting protein prenylation for cancer therapy. Nat Rev Cancer 11(11):775–791
Li L, Zhang W, Cheng S, Cao D, Parent M (2012) Isoprenoids and related pharmacological interventions: potential application in Alzheimer's disease. Mol Neurobiol 46(1):64–77. doi:10.1007/s12035-012-8253-1
Rodriguez-Viciana P, Sabatier C, McCormick F (2004) Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol Cell Biol 24(11):4943–4954. doi:10.1128/MCB.24.11.4943-4954.2004
Du W, Lebowitz PF, Prendergast GC (1999) Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol Cell Biol 19(3):1831–1840
Liu A, Du W, Liu JP, Jessell TM, Prendergast GC (2000) RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol Cell Biol 20(16):6105–6113
ten Klooster JP, Hordijk PL (2007) Targeting and localized signalling by small GTPases. Biol Cell/Under Auspices Eur Cell Biol Organ 99(1):1–12. doi:10.1042/BC20060071
Tolias KF, Duman JG, Um K (2011) Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol 94(2):133–148
Schmidt A, Hall A (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16(13):1587–1609. doi:10.1101/gad.1003302
Bernards A, Settleman J (2004) GAP control: regulating the regulators of small GTPases. Trends Cell Biol 14(7):377–385. doi:10.1016/j.tcb.2004.05.003
Ridley AJ (2001) Rho proteins: linking signaling with membrane trafficking. Traffic 2(5):303–310
Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA (2010) Synaptic plasticity, a symphony in GEF. ACS Chem Neurosci 1(5):348–365. doi:10.1021/cn100012x
Luo ZG, Je HS, Wang Q, Yang F, Dobbins GC, Yang ZH, Xiong WC, Lu B, Mei L (2003) Implication of geranylgeranyltransferase I in synapse formation. Neuron 40(4):703–717
Ye X, Carew TJ (2010) Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron 68(3):340–361
Hughes JR (1958) Post-tetanic potentiation. Physiol Rev 38(1):91–113
Gerrow K, Triller A (2010) Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol 20(5):631–639. doi:10.1016/j.conb.2010.06.010
Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284(5421):1811–1816
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51(1):7–61
Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307(5950):462–465
Premkumar LS, Auerbach A (1996) Identification of a high affinity divalent cation binding site near the entrance of the NMDA receptor channel. Neuron 16(4):869–880
Caporale N, Dan Y (2008) Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 31:25–46. doi:10.1146/annurev.neuro.31.060407.125639
Lisman J (1994) The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci 17(10):406–412
Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276(5321):2042–2045
Mammen AL, Kameyama K, Roche KW, Huganir RL (1997) Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272(51):32528–32533
Sweatt JD (1999) Toward a molecular explanation for long-term potentiation. Learn Mem 6(5):399–416
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294(5544):1030–1038
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44(1):5–21
Bos JL (1989) ras oncogenes in human cancer: a review. Cancer Res 49(17):4682–4689
Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG (2007) Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov 6(7):541–555. doi:10.1038/nrd2221
Stornetta RL, Zhu JJ (2011) Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist 17(1):54–78. doi:10.1177/1073858410365562
Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, Kucherlapati R, Jacks T, Silva AJ (2002) Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415(6871):526–530
Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, Cannon TD, Silva AJ (2005) The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol 15(21):1961–1967
Thornton C, Yaka R, Dinh S, Ron D (2003) H-Ras modulates N-methyl-d-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem 278(26):23823–23829
Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M (2000) Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci 20(7):2504–2511
Adamson P, Marshall CJ, Hall A, Tilbrook PA (1992) Post-translational modifications of p21rho proteins. J Biol Chem 267(28):20033–20038
Baron R, Fourcade E, Lajoie-Mazenc I, Allal C, Couderc B, Barbaras R, Favre G, Faye JC, Pradines A (2000) RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody. Proc Natl Acad Sci U S A 97(21):11626–11631. doi:10.1073/pnas.97.21.11626
Solski PA, Helms W, Keely PJ, Su L, Der CJ (2002) RhoA biological activity is dependent on prenylation but independent of specific isoprenoid modification. Cell Growth Differ : Mol Biol J Am Assoc Cancer Res 13(8):363–373
Govek EE, Hatten ME, Van Aelst L (2011) The role of Rho GTPase proteins in CNS neuronal migration. Dev Neurobiol 71(6):528–553
Newey SE, Velamoor V, Govek EE, Van Aelst L (2005) Rho GTPases, dendritic structure, and mental retardation. J Neurobiol 64(1):58–74
Tejada-Simon MV, Villasana LE, Serrano F, Klann E (2006) NMDA receptor activation induces translocation and activation of Rac in mouse hippocampal area CA1. Biochem Biophys Res Commun 343(2):504–512. doi:10.1016/j.bbrc.2006.02.183
Gruart A, Munoz MD, Delgado-Garcia JM (2006) Involvement of the CA3–CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci 26(4):1077–1087. doi:10.1523/JNEUROSCI.2834-05.2006
Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC (2006) TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A 103(27):10444–10449. doi:10.1073/pnas.0603914103
Zhou XP, Wu KY, Liang B, Fu XQ, Luo ZG (2008) TrkB-mediated activation of geranylgeranyltransferase I promotes dendritic morphogenesis. Proc Natl Acad Sci U S A 105(44):17181–17186. doi:10.1073/pnas.0800846105
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20(14):5329–5338
Klyubin I, Cullen WK, Hu NW, Rowan MJ (2012) Alzheimer's disease Abeta assemblies mediating rapid disruption of synaptic plasticity and memory. Mol Brain 5:25. doi:10.1186/1756-6606-5-25
Nomura I, Takechi H, Kato N (2012) Intraneuronally injected amyloid beta inhibits long-term potentiation in rat hippocampal slices. J Neurophysiol 107(9):2526–2531. doi:10.1152/jn.00589.2011
Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8(8):1051–1058
Wang H, Megill A, He K, Kirkwood A, Lee HK (2012) Consequences of inhibiting amyloid precursor protein processing enzymes on synaptic function and plasticity. Neural Plast 2012:272374. doi:10.1155/2012/272374
Pedrini S, Carter TL, Prendergast G, Petanceska S, Ehrlich ME, Gandy S (2005) Modulation of statin-activated shedding of Alzheimer APP ectodomain by ROCK. PLoS Med 2(1):e18
Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R (2005) Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem 280(19):18755–18770
Ostrowski SM, Wilkinson BL, Golde TE, Landreth G (2007) Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem 282(37):26832–26844
Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K (2008) Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. FASEB J 22(1):47–54
Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B (2003) Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science 302(5648):1215–1217. doi:10.1126/science.1090154
Leuchtenberger S, Kummer MP, Kukar T, Czirr E, Teusch N, Sagi SA, Berdeaux R, Pietrzik CU, Ladd TB, Golde TE, Koo EH, Weggen S (2006) Inhibitors of Rho-kinase modulate amyloid-beta (Abeta) secretion but lack selectivity for Abeta42. J Neurochem 96(2):355–365. doi:10.1111/j.1471-4159.2005.03553.x
Wang PL, Niidome T, Akaike A, Kihara T, Sugimoto H (2009) Rac1 inhibition negatively regulates transcriptional activity of the amyloid precursor protein gene. J Neurosci Res 87(9):2105–2114. doi:10.1002/jnr.22039
Boo JH, Sohn JH, Kim JE, Song H, Mook-Jung I (2008) Rac1 changes the substrate specificity of gamma-secretase between amyloid precursor protein and Notch1. Biochem Biophys Res Commun 372(4):913–917. doi:10.1016/j.bbrc.2008.05.153
Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE (2005) Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med 11(5):545–550
Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschape JA, De Strooper B, Muller U, Shen J, Hartmann T (2005) Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol 7(11):1118–1123
Grimm MO, Rothhaar TL, Hartmann T (2012) The role of APP proteolytic processing in lipid metabolism. Exp Brain Res 217(3–4):365–375
Eckert GP, Hooff GP, Strandjord DM, Igbavboa U, Volmer DA, Muller WE, Wood WG (2009) Regulation of the brain isoprenoids farnesyl- and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol Dis 35(2):251–257
Gartner U, Holzer M, Arendt T (1999) Elevated expression of p21ras is an early event in Alzheimer's disease and precedes neurofibrillary degeneration. Neuroscience 91(1):1–5
Gartner U, Holzer M, Heumann R, Arendt T (1995) Induction of p21ras in Alzheimer pathology. Neuroreport 6(10):1441–1444
Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM (2006) Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci 9(2):234–242. doi:10.1038/nn1630
Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM (2008) p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem 283(20):14132–14143. doi:10.1074/jbc.M708034200
Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH (2008) The beta-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain 131(Pt 1):90–108
Mohamed A, Saavedra L, Di Pardo A, Sipione S, Posse de Chaves E (2012) Beta-amyloid inhibits protein prenylation and induces cholesterol sequestration by impairing SREBP-2 cleavage. J Neurosci 32(19):6490–6500. doi:10.1523/JNEUROSCI.0630-12.2012
Cordle A, Landreth G (2005) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors attenuate beta-amyloid-induced microglial inflammatory responses. J Neurosci 25(2):299–307
Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G (2005) Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem 280(40):34202–34209
Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, Schubert-Zsilavecz M, Karas M, Muller WE, Wood WG (2005) Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther 312(2):786–793
Franke C, Noldner M, Abdel-Kader R, Johnson-Anuna LN, Gibson Wood W, Muller WE, Eckert GP (2007) Bcl-2 upregulation and neuroprotection in guinea pig brain following chronic simvastatin treatment. Neurobiol Dis 25(2):438–445
Cespedes-Rubio A, Jurado FW, Cardona-Gomez GP (2010) p120 catenin/alphaN-catenin are molecular targets in the neuroprotection and neuronal plasticity mediated by atorvastatin after focal cerebral ischemia. J Neurosci Res 88(16):3621–3634
Lee M, You HJ, Cho SH, Woo CH, Yoo MH, Joe EH, Kim JH (2002) Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to beta-amyloid in C6 astroglioma cells. Biochem J 366(Pt 3):937–943
Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M (2008) Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci 28(46):12039–12051
Hamano T, Yen SH, Gendron T, Ko LW, Kuriyama M (2012) Pitavastatin decreases tau levels via the inactivation of Rho/ROCK. Neurobiol Aging 33(10):2306–2320. doi:10.1016/j.neurobiolaging.2011.10.020
Mans RA, Chowdhury N, Cao D, McMahon LL, Li L (2010) Simvastatin enhances hippocampal long-term potentiation in C57BL/6 mice. Neuroscience 166(2):435–444
Mans RA, McMahon LL, Li L (2012) Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience 202:1–9
Li L, Cao D, Kim H, Lester R, Fukuchi K (2006) Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol 60(6):729–739
Cheng S, Cao D, Hottman DA, Yuan L, Bergo MO, Li L (2013) Farnesyltransferase haplodeficiency reduces neuropathology and rescues cognitive function in a mouse model of Alzheimer disease. J Biol Chem 288(50):35952–35960. doi:10.1074/jbc.M113.503904
Shepardson NE, Shankar GM, Selkoe DJ (2010) Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol 68(11):1385–1392
Shepardson NE, Shankar GM, Selkoe DJ (2010) Cholesterol level and statin use in Alzheimer disease: I. Review of epidemiological and preclinical studies. Arch Neurol 68(10):1239–1244
Lansbury PT Jr, Justman CJ, Grammatopoulos TN, Lynch BA, Liu Z (2010) Treatment of proteinopathies using a farnesyl transferase inhibitor. U S Pat 20100160372
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AG031846), the Alzheimer’s Association (IIRG-09-131791), the BrightFocus Foundation (formerly American Health Assistance Foundation) (A2010328), the Alzheimer’s Drug Discovery Foundation, and the Academic Health Center of the University of Minnesota.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hottman, D.A., Li, L. Protein Prenylation and Synaptic Plasticity: Implications for Alzheimer’s Disease. Mol Neurobiol 50, 177–185 (2014). https://doi.org/10.1007/s12035-013-8627-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8627-z