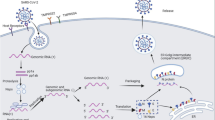
Abstract
SARS-CoV-2 mediated infection instigated a scary pandemic state since 2019. They created havoc comprising death, imbalanced social structures, and a wrecked global economy. During infection, the inflammation and associated cytokine storm generate a critical pathological situation in the human body, especially in the lungs. By the passage of time of infection, inflammatory disorders, and multiple organ damage happen which might lead to death, if not treated properly. Until now, many pathological parameters have been used to understand the progress of the severity of COVID-19 but with limited success. Bioactive lipid mediators have the potential of initiating and resolving inflammation in any disease. The connection between lipid storm and inflammatory states of SARS-CoV-2 infection has surfaced and got importance to understand and mitigate the pathological states of COVID-19. As the role of eicosanoids in COVID-19 infection is not well defined, available information regarding this issue has been accumulated to address the possible network of eicosanoids related to the initiation of inflammation, promotion of cytokine storm, and resolution of inflammation, and highlight possible strategies for treatment and drug discovery related to SARS-CoV-2 infection in this study. Understanding the involvement of eicosanoids in exploration of cellular events provoked by SARS-CoV-2 infection has been summarized as an important factor to deescalate any upcoming catastrophe imposed by the lethal variants of this micro-monster. Additionally, this study also recognized the eicosanoid based drug discovery, treatment, and strategies for managing the severity of SARS-COV-2 infection.
Graphical Abstract





Similar content being viewed by others
Abbreviations
- 15d-PGJ2 :
-
15-Deoxy-delta-12,14-prostaglandin J2
- AA:
-
Arachidonic acid
- ACE 2:
-
Angiotensin-converting enzyme 2
- AKI:
-
Acute kidney injury
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- ATF6:
-
Activating transcription factor 6
- BAL:
-
Bronchoalveolar lavages
- BLT1:
-
B leukotriene subtype 1 receptor
- BLT2:
-
B leukotriene subtype 2 receptor
- CCL2:
-
Chemokine ligand 2 (also known as monocyte chemoattractant protein-1, MCP-1)
- CHOP:
-
CCAAT-enhancer-binding protein homologous protein
- COX-2:
-
Cyclooxygenase-2
- cPGES:
-
Cytosolic prostaglandin E synthase
- cPLA2 :
-
Cytosolic Phospholipase A2
- CXCL10:
-
C-X-C motif chemokine ligand 10 (also known as Interferon gamma-induced protein 10 (IP-10))
- CYP:
-
Cytochrome P450
- CYP4F3:
-
Cytochrome P450 4F3
- CysLTs:
-
Cysteinyl leukotrienes
- DGLA:
-
Dihomo-γ-linolenic acid
- DHA:
-
Docosahexaenoic acid
- DHET:
-
Dihydroxyeicosatrienoic acid
- DP1:
-
Prostaglandin D2 receptor 1
- DP2:
-
Prostaglandin D2 receptor 2
- DPA:
-
Docosapentaenoic acid
- EET:
-
Epoxyeicosatrienoic acid
- eLOX3:
-
Epidermis-type lipoxygenase 3
- EP3:
-
Prostaglandin E2 receptor EP3 subtype
- EP4:
-
Prostaglandin E2 receptor EP4 subtype
- EPA:
-
Eicosapentaenoic acid
- ER:
-
Endoplasmic reticulum
- EX:
-
Eoxin
- FLAP:
-
5-Lipoxygenase-activating protein
- GCSF:
-
Granulocyte colony-stimulating factor
- GGT:
-
Gamma-glutamyl transferase
- 12HDH/15oPGR:
-
12-Hydroxydehydrogenase/15-oxo-prostaglandin-13-reductase
- HDHA:
-
Hydroxy docosahexaenoic acid
- HDPA:
-
Hydroxydocosapentaenoic acid
- HEPE:
-
Hydroxyeicosapentaenoic acid
- HETE:
-
Hydroxyeicosatetraenoic acid
- HETE:
-
Hydroxyeicosatetraenoic acid
- HETrE:
-
Hydroxyeicosatrienoic acid
- HODE:
-
Hydroxyloctadecadienoic acids
- HOTrE:
-
Hydroxyoctadecatrienoic acid
- HpDHA:
-
Hydro(peroxy)-docosahexaenoic acid
- HpEPE:
-
Hydroperoxy-eicosapentaenoic acid
- HPETE:
-
Hydroperoxyeicosatetraenoic acid
- HPMVEC:
-
Human pulmonary microvascular endothelial cell
- HX:
-
Hepoxilin
- HXS:
-
Hepoxilin synthase
- IFNγ:
-
Interferon gamma
- IL:
-
Interleukin
- IL1B:
-
Interleukin 1 beta
- IP-10:
-
Interferon gamma-induced protein 10 (also known as CXCL10, C-X-C motif chemokine ligand 10)
- IRE1:
-
Inositol-requiring enzyme 1
- LA:
-
Linoleic acid
- LOX:
-
Lipoxygenase
- LTA4 :
-
Leukotriene A4
- LTA4H:
-
LTA4 hydrolase
- LTB4 :
-
Leukotriene B4
- LTC4 :
-
Leukotriene C4
- LTD4 :
-
Leukotriene D4
- LTE4 :
-
Leukotriene E4
- LXA4 :
-
Lipoxin A4
- LXB4 :
-
Lipoxin B4
- MBD:
-
Membrane bound dipeptidase
- MCP-1:
-
Monocyte chemoattractant protein-1 (also known as CCL2, chemokine ligand 2)
- MDSC:
-
Myeloid monocyte-derived suppressor cells
- MERS-CoV:
-
Middle east respiratory syndrome coronavirus
- MIP1a:
-
Macrophage inflammatory protein 1 alpha
- mPGES:
-
Microsomal prostaglandin E synthase
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAID:
-
Nonsteroidal anti-inflammatory drugs
- p38 MAPK:
-
P38 mitogen-activated kinase
- PD1:
-
Protectin D1
- PDX:
-
Protectin DX
- PERK:
-
Protein kinase R-like ER kinase
- PG:
-
Prostaglandins
- PGD2 :
-
Prostaglandin D2
- PGDKR:
-
PGD2-11-keto reductase
- PGDS:
-
Prostaglandin D synthase
- PGE2 :
-
Prostaglandin E2
- PGES:
-
Prostaglandin E synthase
- PGF2α :
-
Prostaglandin F2α
- PGFS:
-
Prostaglandin F2α synthase
- PGH2 :
-
Prostaglandin H2
- PGI2 :
-
Prostaglandin I2 (prostacyclin)
- PGIS:
-
Prostaglandin I2 synthase
- PGJ2 :
-
Prostaglandin J2
- PI3K:
-
Phosphoinositide 3-kinase
- PKA:
-
CAMP-dependent protein kinase
- PLA2 :
-
Phospholipase A2
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- ROS:
-
Reactive oxygen species
- Rv:
-
Resolvin
- S protein:
-
Spike protein
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- sEH:
-
Soluble epoxide hydrolase
- SPM:
-
Specialized proresolving mediators
- TMPRSS2:
-
Transmembrane serine protease 2
- TNFα:
-
Tumor necrosis factor alpha
- TP:
-
Thromboxane A2 receptor
- TXA2 :
-
Thromboxane A2
- TXAS:
-
Thromboxane A2 synthase
- TXB2 :
-
Thromboxane B2
- UPR:
-
Unfolded-protein response
- XBP1:
-
X box-binding protein 1
References
Huang, Y., Yang, C., Xu, X. F., Xu, W., & Liu, S. W. (2020). Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica., 41(9), 1141–1149. https://doi.org/10.1038/s41401-020-0485-4
Duong, D. (2021). Alpha, Beta, Delta, Gamma What’s important to know about SARS-CoV-2 variants of concern? Canadian Medical Association Journal., 193(27), E1059–E1060. https://doi.org/10.1503/cmaj.1095949
Rahman, S., Montero, M. T. V., Rowe, K., Kirton, R., & Kunik, F., Jr. (2021). Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert Review of Clinical Pharmacology, 14(5), 601–621. https://doi.org/10.1080/17512433.2021.1902303
Abdullah, F., Myers, J., Basu, D., Tintinger, G., Ueckermann, V., Mathebula, M., et al. (2022). Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. International Journal of Infectious Diseases, 116, 38–42. https://doi.org/10.1016/j.ijid.2021.12.357
Coperchini, F., Chiovato, L., Croce, L., Magri, F., & Rotondi, M. (2020). The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine & Growth Factor Reviews., 53, 25–32. https://doi.org/10.1016/j.cytogfr.2020.05.003
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Bhatia, M., Zemans, R. L., & Jeyaseelan, S. (2012). Role of chemokines in the pathogenesis of acute lung injury. American Journal of Respiratory Cell and Molecular Biology., 46(5), 566–572. https://doi.org/10.1165/rcmb.2011-0392TR
Ye, Q., Wang, B., & Mao, J. (2020). The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. Journal of Infection, 80(6), 607–613. https://doi.org/10.1016/j.jinf.2020.03.037
Channappanavar, R., Fehr, A. R., Vijay, R., Mack, M., Zhao, J., Meyerholz, D. K., et al. (2016). Dysregulated Type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe., 19(2), 181–193. https://doi.org/10.1016/j.chom.2016.01.007
Herold, S., Steinmueller, M., von Wulffen, W., Cakarova, L., Pinto, R., Pleschka, S., et al. (2008). Lung epithelial apoptosis in influenza virus pneumonia: The role of macrophage-expressed TNF-related apoptosis-inducing ligand. Journal of Experimental Medicine., 205(13), 3065–3077. https://doi.org/10.1084/jem.20080201
Hogner, K., Wolff, T., Pleschka, S., Plog, S., Gruber, A. D., Kalinke, U., et al. (2013). Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLOS Pathogens., 9(2), e1003188. https://doi.org/10.1371/journal.ppat.1003188
Rodrigue-Gervais, I. G., Labbe, K., Dagenais, M., Dupaul-Chicoine, J., Champagne, C., Morizot, A., et al. (2014). Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host & Microbe, 15(1), 23–35. https://doi.org/10.1016/j.chom.2013.12.003
Hammock, B. D., Wang, W., Gilligan, M. M., & Panigrahy, D. (2020). Eicosanoids: The Overlooked Storm in Coronavirus Disease 2019 (COVID-19)? The American Journal of Pathology., 190(9), 1782–1788. https://doi.org/10.1016/j.ajpath.2020.06.010
Jorgensen, I., Rayamajhi, M., & Miao, E. A. (2017). Programmed cell death as a defence against infection. Nature Reviews Immunology., 17(3), 151–164. https://doi.org/10.1038/nri.2016.147
Gartung, A., Yang, J., Sukhatme, V. P., Bielenberg, D. R., Fernandes, D., Chang, J., et al. (2019). Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proceedings of the National Academy of Sciences of the United States of America., 116(5), 1698–1703. https://doi.org/10.1073/pnas.1803999116
Channappanavar, R., & Perlman, S. (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars in Immunopathology., 39(5), 529–539. https://doi.org/10.1007/s00281-017-0629-x
Scheller, J., & Rose-John, S. (2006). Interleukin-6 and its receptor: From bench to bedside. Medical Microbiology and Immunology, 195(4), 173–183. https://doi.org/10.1007/s00430-006-0019-9
Smits, S. L., de Lang, A., van den Brand, J. M., Leijten, L. M., van, I.W.F., Eijkemans, M, J., et al. (2010). Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathogens., 6(2), e1000756. https://doi.org/10.1371/journal.ppat.1000756
Panigrahy, D., Gilligan, M. M., Huang, S., Gartung, A., Cortes-Puch, I., Sime, P. J., et al. (2020). Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer and Metastasis Reviews, 39(2), 337–340. https://doi.org/10.1007/s10555-020-09889-4
Schroder, M., & Kaufman, R. J. (2005). The mammalian unfolded protein response. Annual Review of Biochemistry, 74, 739–789. https://doi.org/10.1146/annurev.biochem.73.011303.074134
Chan, C. P., Siu, K. L., Chin, K. T., Yuen, K. Y., Zheng, B., & Jin, D. Y. (2006). Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. Journal of Virology., 80(18), 9279–9287. https://doi.org/10.1128/JVI.00659-06
Versteeg, G. A., van de Nes, P. S., Bredenbeek, P. J., & Spaan, W. J. (2007). The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. Journal of Virology, 81(20), 10981–10990. https://doi.org/10.1128/JVI.01033-07
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T., & Mori, K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell, 107(7), 881–891. https://doi.org/10.1016/s0092-8674(01)00611-0
Lee, A. H., Iwakoshi, N. N., & Glimcher, L. H. (2003). XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and Cellular Biology., 23(21), 7448–7459. https://doi.org/10.1128/MCB.23.21.7448-7459.2003
Chopra, S., Giovanelli, P., Alvarado-Vazquez, P. A., Alonso, S., Song, M., Sandoval, T. A., et al. (2019). IRE1alpha-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science. https://doi.org/10.1126/science.aau6499
Shi, C. S., Nabar, N. R., Huang, N. N., & Kehrl, J. H. (2019). SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov., 5, 101. https://doi.org/10.1038/s41420-019-0181-7
Liu, M., Gu, C., Wu, J., & Zhu, Y. (2006). Amino acids 1 to 422 of the spike protein of SARS associated coronavirus are required for induction of cyclooxygenase-2. Virus Genes, 33(3), 309–317. https://doi.org/10.1007/s11262-005-0070-4
Yan, X., Hao, Q., Mu, Y., Timani, K. A., Ye, L., Zhu, Y., et al. (2006). Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. International Journal of Biochemistry & Cell Biology, 38(8), 1417–1428. https://doi.org/10.1016/j.biocel.2006.02.003
Schmelzer, K. R., Kubala, L., Newman, J. W., Kim, I. H., Eiserich, J. P., & Hammock, B. D. (2005). Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A., 102(28), 9772–9777. https://doi.org/10.1073/pnas.0503279102
von Moltke, J., Trinidad, N. J., Moayeri, M., Kintzer, A. F., Wang, S. B., van Rooijen, N., et al. (2012). Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature, 490(7418), 107–111. https://doi.org/10.1038/nature11351
Tielemans, B., Stoian, L., Gijsbers, R., Michiels, A., Wagenaar, A., Farre Marti, R., et al. (2019). Cytokines trigger disruption of endothelium barrier function and p38 MAP kinase activation in BMPR2-silenced human lung microvascular endothelial cells. Pulm Circ., 9(4), 2045894019883607. https://doi.org/10.1177/2045894019883607
Huang, K. J., Su, I. J., Theron, M., Wu, Y. C., Lai, S. K., Liu, C. C., et al. (2005). An interferon-gamma-related cytokine storm in SARS patients. Journal of Medical Virology., 75(2), 185–194. https://doi.org/10.1002/jmv.20255
Choo-Wing, R., Syed, M. A., Harijith, A., Bowen, B., Pryhuber, G., Janer, C., et al. (2013). Hyperoxia and interferon-gamma-induced injury in developing lungs occur via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent pathway. American Journal of Respiratory Cell and Molecular Biology., 48(6), 749–757. https://doi.org/10.1165/rcmb.2012-0381OC
Baghaki, S., Yalcin, C. E., Baghaki, H. S., Aydin, S. Y., Daghan, B., & Yavuz, E. (2020). COX2 inhibition in the treatment of COVID-19: Review of literature to propose repositioning of celecoxib for randomized controlled studies. International Journal of Infectious Diseases., 101, 29–32. https://doi.org/10.1016/j.ijid.2020.09.1466
Dennis, E. A., & Norris, P. C. (2015). Eicosanoid storm in infection and inflammation. Nature Reviews Immunology., 15(8), 511–523. https://doi.org/10.1038/nri3859
Serhan, C. N. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature, 510(7503), 92–101. https://doi.org/10.1038/nature13479
Wang, B., Wu, L., Chen, J., Dong, L., Chen, C., Wen, Z., et al. (2021). Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduction and Targeted Therapy, 6(1), 94. https://doi.org/10.1038/s41392-020-00443-w
Rahman, M. S., Khan, F., Syeda, P. K., Nishimura, K., Jisaka, M., Nagaya, T., et al. (2014). Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology, 66(4), 635–646. https://doi.org/10.1007/s10616-013-9616-9
Khan, F., Syeda, P. K., Nartey, M. N., Rahman, M. S., Islam, M. S., Nishimura, K., et al. (2016). Stimulation of fat storage by prostacyclin and selective agonists of prostanoid IP receptor during the maturation phase of cultured adipocytes. Cytotechnology, 68(6), 2417–2429. https://doi.org/10.1007/s10616-016-9960-7
Chowdhury, A. A., Rahman, M. S., Nishimura, K., Jisaka, M., Nagaya, T., Ishikawa, T., et al. (2011). 15-Deoxy-D12,14-prostaglandin J2 interferes inducible synthesis of prostaglandins E2 and F2a that suppress subsequent adipogenesis program in cultured preadipocytes. Prostaglandins & Other Lipid Mediators., 95(1–4), 53–62. https://doi.org/10.1016/j.prostaglandins.2011.06.002
Hossain, M. S., Chowdhury, A. A., Rahman, M. S., Nishimura, K., Jisaka, M., Nagaya, T., et al. (2011). Development of enzyme-linked immunosorbent assay for Δ 12-PG2 and its application to the measurement of the endogenous product generated by cultured adipocytes during the maturation phase. Prostaglandins & Other Lipid Mediators., 94(3–4), 73–80. https://doi.org/10.1016/j.prostaglandins.2010.12.005
Hopkins, N. K., & Gorman, R. R. (1981). Regulation of 3T3-L1 fibroblast differentiation by prostacyclin (prostaglandin I2). Biochimica et Biophysica Acta., 663(2), 457–466. https://doi.org/10.1016/0005-2760(81)90174-0
Xu, L., Miyoshi, H., Nishimura, K., Jisaka, M., Nagaya, T., & Yokota, K. (2007). Gene expression of isoformic enzymes in arachidonate cyclooxygenase pathway and the regulation by tumor necrosis factor alpha during life cycle of adipocytes. Prostaglandins & Other Lipid Mediators, 83(3), 213–218. https://doi.org/10.1016/j.prostaglandins.2007.01.009
Rahman, M. S. (2019). Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. Journal of Cellular Physiology, 234(4), 3254–3262. https://doi.org/10.1002/jcp.26932
Schilling, M., Gassmann, N., Regli, B., Stoupis, C., & Buckler, M. W. (1996). Increased thromboxane B2 and prostaglandin E2 levels precede clinical acute respiratory distress syndrome after esophageal resection. Digestive Surgery, 13(4–5), 273–276.
Slotman, G. J., Burchard, K. W., & Gann, D. S. (1985). Thromboxane and prostacyclin in clinical acute respiratory failure. Journal of Surgical Research, 39(1), 1–7. https://doi.org/10.1016/0022-4804(85)90154-4
Deby-Dupont, G., Braun, M., Lamy, M., Deby, C., Pincemail, J., Faymonville, M. E., et al. (1987). Thromboxane and prostacyclin release in adult respiratory distress syndrome. Intensive Care Medicine., 13(3), 167–174.
D’Elia, R. V., Harrison, K., Oyston, P. C., Lukaszewski, R. A., & Clark, G. C. (2013). Targeting the “cytokine storm” for therapeutic benefit. Clinical and Vaccine Immunology., 20(3), 319–327. https://doi.org/10.1128/CVI.00636-12
Aso, H., Ito, S., Mori, A., Morioka, M., Suganuma, N., Kondo, M., et al. (2012). Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. American Journal of Physiology—Lung Cellular and Molecular Physiology., 302(2), L266–L273. https://doi.org/10.1152/ajplung.00248.2011
Tsuge, K., Inazumi, T., Shimamoto, A., & Sugimoto, Y. (2019). Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. International Immunology, 31(9), 597–606. https://doi.org/10.1093/intimm/dxz021
McLoughlin, R. M., Hurst, S. M., Nowell, M. A., Harris, D. A., Horiuchi, S., Morgan, L. W., et al. (2004). Differential regulation of neutrophil-activating chemokines by IL-6 and its soluble receptor isoforms. Journal of Immunology., 172(9), 5676–5683. https://doi.org/10.4049/jimmunol.172.9.5676
Ricke-Hoch, M., Stelling, E., Lasswitz, L., Gunesch, A. P., Kasten, M., Zapatero-Belinchon, F. J., et al. (2021). Impaired immune response mediated by prostaglandin E2 promotes severe COVID-19 disease. PLoS ONE, 16(8), e0255335. https://doi.org/10.1371/journal.pone.0255335
Nakao, S., Ogtata, Y., Shimizu, E., Yamazaki, M., Furuyama, S., & Sugiya, H. (2002). Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase-2 (COX-2) transcription via NFkappaB in human gingival fibroblasts. Molecular and Cellular Biochemistry, 238(1–2), 11–18. https://doi.org/10.1023/A:1019927616000
Kaneko, N., Kuo, H. H., Boucau, J., Farmer, J. R., Allard-Chamard, H., Mahajan, V. S., et al. (2020). Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. https://doi.org/10.1016/j.cell.2020.08.025
Jandl, K., Stacher, E., Balint, Z., Sturm, E. M., Maric, J., Peinhaupt, M., et al. (2016). Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. Journal of Allergy and Clinical Immunology., 137(3), 833–843. https://doi.org/10.1016/j.jaci.2015.11.012
Gupta, A., Kalantar-Zadeh, K., & Reddy, S. T. (2020). Ramatroban as a novel immunotherapy for COVID-19. Journal of Molecular and Genetic Medicine. https://doi.org/10.37421/jmgm.2020.14.457
Cloutier, A., Marois, I., Cloutier, D., Verreault, C., Cantin, A. M., & Richter, M. V. (2012). The prostanoid 15-deoxy-Δ12,14-prostaglandin-j2 reduces lung inflammation and protects mice against lethal influenza infection. The Journal of Infectious Diseases., 205(4), 621–630. https://doi.org/10.1093/infdis/jir804
Hoxha, M. (2020). What about COVID-19 and arachidonic acid pathway? European Journal of Clinical Pharmacology., 76(11), 1501–1504. https://doi.org/10.1007/s00228-020-02941-w
Zhao, J., Zhao, J., Legge, K., & Perlman, S. (2011). Age-related increases in PGD2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. The Journal of Clinical Investigation., 121(12), 4921–4930. https://doi.org/10.1172/JCI59777
Ogletree, M. L., Chander Chiang, K., Kulshrestha, R., Agarwal, A., Agarwal, A., & Gupta, A. (2022). Treatment of COVID-19 pneumonia and acute respiratory distress with ramatroban, a thromboxane A2 and prostaglandin D2 receptor antagonist: A four-patient case series report. Frontiers in Pharmacology, 13, 904020. https://doi.org/10.3389/fphar.2022.904020
Larsson, A. K., Hagfjärd, A., Dahlén, S. E., & Adner, M. (2011). Prostaglandin D2 induces contractions through activation of TP receptors in peripheral lung tissue from the guinea pig. European Journal of Pharmacology, 669(1–3), 136–142. https://doi.org/10.1016/j.ejphar.2011.07.046
Archam bault, A. S., Zaid, Y., Rakotoarivelo, V., Turcotte, C., Dore, E., Dubuc, I., et al. (2021). High levels of eicosanoids and docosanoids in the lungs of intubated COVID-19 patients. FASEB Journal., 35(6), e21666. https://doi.org/10.1096/fj.202100540R
Wong, L. R., Zheng, J., Wilhelmsen, K., Li, K., Ortiz, M. E., Schnicker, N. J., et al. (2022). Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature, 605(7908), 146–151. https://doi.org/10.1038/s41586-022-04630-3
Verma, H., Mendiratta, N., & Tripathi, R. K. (2022). Prostaglandin E1 infusion could improve outcomes in COVID-related limb ischemia—a single operator series of 17 patients. Journal of Vascular Surgery, 75(6), e291–e292. https://doi.org/10.1016/j.jvs.2022.03.658
Shimizu, T., Izumi, T., Seyama, Y., & Tadokoro, K. (1986). Radmark O, Samuelsson B, Characterization of leukotriene A4 synthase from murine mast cells: Evidence for its identity to arachidonate 5-lipoxygenase. Proc Natl Acad Sci U S A., 83(12), 4175–4179. https://doi.org/10.1073/pnas.83.12.4175
Lewis, R. A., Austen, K. F., & Soberman, R. J. (1990). Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. New England Journal of Medicine., 323(10), 645–655. https://doi.org/10.1056/NEJM199009063231006
Peters-Golden, M., Gleason, M. M., & Togias, A. (2006). Cysteinyl leukotrienes: Multi-functional mediators in allergic rhinitis. Clinical and Experimental Allergy, 36(6), 689–703. https://doi.org/10.1111/j.1365-2222.2006.02498.x
Bäck, M., Sultan, A., Ovchinnikova, O., & Hansson, G. K. (2007). 5-Lipoxygenase-activating protein: A potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circulation Research., 100(7), 946–949. https://doi.org/10.1161/01.RES.0000264498.60702.0d
Snelgrove, R. J., Jackson, P. L., Hardison, M. T., Noerager, B. D., Kinloch, A., Gaggar, A., et al. (2010). A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science, 330(6000), 90–94. https://doi.org/10.1126/science.1190594
Wheelan, P., Sala, A., Folco, G., Nicosia, S., Falck, J. R., Bhatt, R. K., et al. (1994). Stereochemical analysis and biological activity of 3-hydroxy-leukotriene B4: A metabolite from ethanol-treated rat hepatocytes. Journal of Pharmacology and Experimental Therapeutics, 271(3), 1514–1519.
Fitzpatrick, F., Haeggstrom, J., Granstrom, E., & Samuelsson, B. (1983). Metabolism of leukotriene A4 by an enzyme in blood plasma: A possible leukotactic mechanism. Proceedings of the National Academy of Sciences of the United States of America., 80(17), 5425–5429. https://doi.org/10.1073/pnas.80.17.5425
Munafo, D. A., Shindo, K., Baker, J. R., & Bigby, T. D. (1994). Leukotriene A4 hydrolase in human bronchoalveolar lavage fluid. Journal of Clinical Investigation., 93(3), 1042–1050. https://doi.org/10.1172/JCI117053
Low, C. M., Akthar, S., Patel, D. F., Loser, S., Wong, C. T., Jackson, P. L., et al. (2017). The development of novel LTA4H modulators to selectively target LTB4 generation. Scientific Reports., 7, 44449. https://doi.org/10.1038/srep44449
Haeggstrom, J. Z. (2004). Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. Journal of Biological Chemistry., 279(49), 50639–50642. https://doi.org/10.1074/jbc.R400027200
Bhatt, L., Roinestad, K., Van, T., & Springman, E. B. (2017). Recent advances in clinical development of leukotriene B4 pathway drugs. Seminars in Immunology., 33, 65–73. https://doi.org/10.1016/j.smim.2017.08.007
Funk, C. D., & Ardakani, A. (2020). A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Frontiers in Pharmacology., 11, 1214. https://doi.org/10.3389/fphar.2020.01214
Watanabe, M., Machida, K., & Inoue, H. (2014). A turn on and a turn off: BLT1 and BLT2 mechanisms in the lung. Expert Review of Respiratory Medicine, 8(4), 381–383. https://doi.org/10.1586/17476348.2014.908715
Aigner, L., Pietrantonio, F., Bessa de Sousa, D. M., Michael, J., Schuster, D., Reitsamer, H. A., et al. (2020). The leukotriene receptor antagonist montelukast as a potential COVID-19 therapeutic. Frontiers in Molecular Biosciences., 7, 610132. https://doi.org/10.3389/fmolb.2020.610132
Capra, V. (2004). Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharmacological Research., 50(1), 1–11. https://doi.org/10.1016/j.phrs.2003.12.012
Duah, E., Adapala, R. K., Al-Azzam, N., Kondeti, V., Gombedza, F., Thodeti, C. K., et al. (2013). Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Scientific Reports., 3, 3274. https://doi.org/10.1038/srep03274
Kanaoka, Y., & Boyce, J. A. (2004). Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. Journal of Immunology., 173(3), 1503–1510. https://doi.org/10.4049/jimmunol.173.3.1503
Maeba, S., Ichiyama, T., Ueno, Y., Makata, H., Matsubara, T., & Furukawa, S. (2005). Effect of montelukast on nuclear factor kappaB activation and proinflammatory molecules. Annals of Allergy, Asthma & Immunology., 94(6), 670–674. https://doi.org/10.1016/S1081-1206(10)61326-9
Auner, B., Geiger, E. V., Henrich, D., Lehnert, M., Marzi, I., & Relja, B. (2012). Circulating leukotriene B4 identifies respiratory complications after trauma. Mediators of Inflammation., 2012, 536156. https://doi.org/10.1155/2012/536156
Takahashi, G., Shibata, S., & Endo, S. (2017). Eicosanoids as risk and prognostic factors for acute respiratory distress syndrome in sepsis patients. J Pulm Respir Med., 7(6), 435. https://doi.org/10.4172/2161-105X.1000435
Amat, M., Barcons, M., Mancebo, J., Mateo, J., Oliver, A., Mayoral, J.-F., et al. (2000). Evolution of leukotriene B4, peptide leukotrienes, and interleukin-8 plasma concentrations in patients at risk of acute respiratory distress syndrome and with acute respiratory distress syndrome: Mortality prognostic study. Critical Care Medicine., 28(1), 57–62. https://doi.org/10.1097/00003246-200001000-00009
Westcott, J. Y., Thomas, R. B., & Voelkel, N. F. (1991). Elevated urinary leukotriene E4 excretion in patients with ARDS and severe burns. Prostaglandins Leukotrienes and Essential Fatty Acids, 43(3), 151–158. https://doi.org/10.1016/0952-3278(91)90162-x
Werz, O., & Steinhilber, D. (2006). Therapeutic options for 5-lipoxygenase inhibitors. Pharmacology & Therapeutics, 112(3), 701–718. https://doi.org/10.1016/j.pharmthera.2006.05.009
Tsai, M. J., Chang, W. A., Tsai, P. H., Wu, C. Y., Ho, Y. W., Yen, M. C., et al. (2017). Montelukast induces apoptosis-inducing factor-mediated cell death of lung cancer cells. International Journal of Molecular Science. https://doi.org/10.3390/ijms18071353
Spector, A. A. (2009). Arachidonic acid cytochrome P450 epoxygenase pathway. Journal of Lipid Research, 50(Suppl), S52–S56. https://doi.org/10.1194/jlr.R800038-JLR200
Tacconelli, S., & Patrignani, P. (2014). Inside epoxyeicosatrienoic acids and cardiovascular disease. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2014.00239
Gilroy, D. W., Edin, M. L., De Maeyer, R. P., Bystrom, J., Newson, J., Lih, F. B., et al. (2016). CYP450-derived oxylipins mediate inflammatory resolution. Proceedings of the National Academy of Sciences of the United States of America., 113(23), E3240–E3249. https://doi.org/10.1073/pnas.1521453113
Thomson, S. J., Askari, A., & Bishop-Bailey, D. (2012). Anti-inflammatory effects of epoxyeicosatrienoic acids. International Journal of Vascular Medicine., 2012, 605101. https://doi.org/10.1155/2012/605101
Deng, Y., Edin, M. L., Theken, K. N., Schuck, R. N., Flake, G. P., Kannon, M. A., et al. (2011). Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB Journal., 25(2), 703–713. https://doi.org/10.1096/fj.10-171488
Yu, G., Zeng, X., Wang, H., Hou, Q., Tan, C., Xu, Q., et al. (2015). 14,15-epoxyeicosatrienoic acid suppresses cigarette smoke extract-induced apoptosis in lung epithelial cells by inhibiting endoplasmic reticulum stress. Cellular Physiology and Biochemistry., 36(2), 474–486. https://doi.org/10.1159/000430113
Wang, D., & Dubois, R. N. (2012). Epoxyeicosatrienoic acids: A double-edged sword in cardiovascular diseases and cancer. The Journal of Clinical Investigation, 122(1), 19–22. https://doi.org/10.1172/JCI61453
Bellien, J., & Joannides, R. (2013). Epoxyeicosatrienoic acid pathway in human health and diseases. Journal of Cardiovascular Pharmacology., 61(3), 188–196. https://doi.org/10.1097/FJC.0b013e318273b007
Norris, P. C., Gosselin, D., Reichart, D., Glass, C. K., & Dennis, E. A. (2014). Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A., 111(35), 12746–12751. https://doi.org/10.1073/pnas.1404372111
Snider, J. M., You, J. K., Wang, X., Snider, A. J., Hallmark, B., Zec, M. M., et al. (2021). Group IIA secreted phospholipase A2 is associated with the pathobiology leading to COVID-19 mortality. Journal of Clinical Investigation. https://doi.org/10.1172/JCI149236
Kuypers, F. A., Rostad, C. A., Anderson, E. J., Chahroudi, A., Jaggi, P., Wrammert, J., et al. (2021). Secretory phospholipase A2 in SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C). Experimental Biology and Medicine., 246(23), 2543–2552. https://doi.org/10.1177/15353702211028560
Trostchansky, A., Souza, J. M., Ferreira, A., Ferrari, A., Blanco, F., & Trujillo, M. (2007). Synthesis, isomer characterization, and anti-Inflammatory properties of nitroarachidonate. Biochemistry, 46(15), 45–53.
Baral, P. K., Amin, M. T., Rashid, M. M. O., & Hossain, M. S. (2022). Assessement of polyunsaturated fatty acids on COVID-19 associated risk reduction. Brazilian Journal of Pharmacognosy., 32, 50–64. https://doi.org/10.1007/s43450-021-00213-x
Ripon, M. A. R., Bhowmik, D. R., Amin, M. T., & Hossain, M. S. (2021). Role of Arachidonic cascade in COVID-19 infection. Prostaglandin and Other lipid mediators., 154, 106539. https://doi.org/10.1016/j.prostaglandins.2021.106539
Beigel, J. H., Tomashek, K. M., Dodd, L. E., Metha, A. K., et al. (2022). Remidisivir for the traetment of COVID-19, Final Report. New Enland Journal of Medicine., 383, 1813–1826.
Navabshan, I., Sakthivel, B., Pandiyan, R., Antoniraj, M. G., Dharmaraj, S., Ashokkumar, V., Khoo, K.S., Chew, K.W,. Sugumaran, A., Show, P.L. (2021) Computational lock and key and dynamic trajectory analysis of natural biophors against COVID-19 spike protein to identify effective lead molecules. Mol Biotechnol. Oct;63(10):898–908. doi: https://doi.org/10.1007/s12033-021-00358-z. Epub 2021 Jun 22. Erratum in: Mol Biotechnol. 2021 Aug 10;: PMID: 34159564; PMCID: PMC8219180.
Park, J. H., Park, E. B., Lee, J. Y., & Min, J. Y. (2016). Identification of novel membrane-associated prostaglandin E synthase-1 (mPGES-1) inhibitors with anti-influenza activities in vitro. Biochemical and Biophysical Research Communications, 469(4), 848–855. https://doi.org/10.1016/j.bbrc.2015.11.129
Gross, S., Tilly, P., Hentsch, D., Vonesch, J. L., & Fabre, J. E. (2007). Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors. Journal of Experimental Medicine., 204(2), 311–320. https://doi.org/10.1084/jem.20061617
Janssen, M. C. H., Koene, S., de Laat, P., Hemelaar, P., Pickkers, P., Spaans, E., et al. (2019). The KHENERGY Study: Safety and Efficacy of KH176 in Mitochondrial m.3243A>G Spectrum Disorders. Clinical Pharmacology & Therapeutics., 105(1), 101–111. https://doi.org/10.1002/cpt.1197
Beyrath, J., Pellegrini, M., Renkema, H., Houben, L., Pecheritsyna, S., van Zandvoort, P., et al. (2018). KH176 safeguards mitochondrial diseased cells from redox stress-induced cell death by interacting with the thioredoxin system/peroxiredoxin enzyme machinery. Scientific Reports., 8(1), 6577. https://doi.org/10.1038/s41598-018-24900-3
Liu, J., Wang, Y., Johnson, M. G., Li, A.-R., Shen, W., Wang, X., et al. (2012). Optimization of phenylacetic acid derivatives for balanced CRTH2 and DP dual antagonists. Bioorganic & Medicinal Chemistry Letters., 22(4), 1686–1689. https://doi.org/10.1016/j.bmcl.2011.12.107
Sala, A., Murphy, R. C., & Voelkel, N. F. (1991). Direct airway injury results in elevated levels of sulfidopeptide leukotrienes, detectable in airway secretions. Prostaglandins, 42(1), 1–7. https://doi.org/10.1016/0090-6980(91)90088-w
Bernard, G. R., Korley, V., Chee, P., Swindell, B., Ford-Hutchinson, A. W., & Tagari, P. (1991). Persistent generation of peptido leukotrienes in patients with the adult respiratory distress syndrome. The American Review of Respiratory Disease., 144(2), 263–267. https://doi.org/10.1164/ajrccm/144.2.263
Wu, C., Liu, Y., Yang, Y., Zhang, P., Zhong, W., Wang, Y., et al. (2020). Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B., 10(5), 766–788. https://doi.org/10.1016/j.apsb.2020.02.008
Almerie, M. Q., & Kerrigan, D. D. (2020). The association between obesity and poor outcome after COVID-19 indicates a potential therapeutic role for montelukast. Medical Hypotheses., 143, 109883. https://doi.org/10.1016/j.mehy.2020.109883
Schunck, W. H., Konkel, A., Fischer, R., & Weylandt, K. H. (2018). Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacology & Therapeutics, 183, 177–204. https://doi.org/10.1016/j.pharmthera.2017.10.016
Lopez-Vicario, C., Alcaraz-Quiles, J., Garcia-Alonso, V., Rius, B., Hwang, S. H., Titos, E., et al. (2015). Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: Role for omega-3 epoxides. Proceedings of the National Academy of Sciences of the United States of America., 112(2), 536–541. https://doi.org/10.1073/pnas.14225901
Xia, R., Sun, L., Liao, J., Li, H., You, X., Xu, D., et al. (2019). Inhibition of pancreatic carcinoma growth through enhancing ω-3 epoxy polyunsaturated fatty acid profile by inhibition of soluble epoxide hydrolase. Anticancer Research., 39(7), 3651–3660. https://doi.org/10.21873/anticanres.13513
Zeldin, D. C. (2001). Epoxygenase pathways of arachidonic acid metabolism. Journal of Biological Chemistry, 276(39), 36059–36062. https://doi.org/10.1074/jbc.R100030200
Imig, J. D., & Hammock, B. D. (2009). Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nature Reviews Drug Discovery., 8(10), 794–805. https://doi.org/10.1038/nrd2875
Zhou, Y., Liu, T., Duan, J. X., Li, P., Sun, G. Y., Liu, Y. P., et al. (2017). Soluble epoxide hydrolase inhibitor attenuates lipopolysaccharide-induced acute lung injury and improves survival in mice. Shock, 47(5), 638–645. https://doi.org/10.1097/SHK.0000000000000767
Ono, E., Dutile, S., Kazani, S., Wechsler, M. E., Yang, J., Hammock, B. D., et al. (2014). Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. American Journal of Respiratory and Critical Care Medicine., 190(8), 886–897. https://doi.org/10.1164/rccm.201403-0544OC
Goswami, S. K., Wan, D., Yang, J., Trindade da Silva, C. A., Morisseau, C., Kodani, S. D., et al. (2016). Anti-ulcer efficacy of soluble epoxide hydrolase inhibitor TPPU on diclofenac-induced intestinal ulcers. The Journal of Pharmacology and Experimental Therapeutics., 357(3), 529–536. https://doi.org/10.1124/jpet.116.232108
Ghosh, R., Goswami, S. K., Feitoza, L., Hammock, B., & Gomes, A. V. (2016). Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. International Journal of Cardiology., 223, 923–935. https://doi.org/10.1016/j.ijcard.2016.08.233
Panigrahy, D., Gartung, A., Yang, J., Yang, H., Gilligan, M. M., Sulciner, M. L., et al. (2019). Preoperative stimulation of resolution and inflammation blockade eradicates micrometastases. The Journal of Clinical Investigation, 129(7), 2964–2979. https://doi.org/10.1172/JCI127282
Barden, A. E., Mas, E., & Mori, T. A. (2016). n-3 Fatty acid supplementation and proresolving mediators of inflammation. Current Opinion in Lipidology., 27(1), 26–32. https://doi.org/10.1097/MOL.0000000000000262
Souza, P. R., Marques, R. M., Gomez, E. A., Colas, R. A., De Matteis, R., Zak, A., et al. (2020). Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circulation Research., 126(1), 75–90. https://doi.org/10.1161/CIRCRESAHA.119.315506
Norris, P. C., Arnardottir, H., Sanger, J. M., Fichtner, D., Keyes, G. S., & Serhan, C. N. (2018). Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins, Leukotrienes and Essential Fatty Acids., 138, 81–89. https://doi.org/10.1016/j.plefa.2016.01.001
Spite, M., Norling, L. V., Summers, L., Yang, R., Cooper, D., Petasis, N. A., et al. (2009). Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature, 461(7268), 1287–1291. https://doi.org/10.1038/nature08541
Dalli, J., Chiang, N., & Serhan, C. N. (2015). Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nature medicine., 21(9), 1071–1075. https://doi.org/10.1038/nm.3911
Arnardottir, H., Pawelzik, S. C., Ohlund, W., U., Artiach, G, Hofmann, R., Reinholdsson, I., et al. (2020). Stimulating the resolution of inflammation through omega-3 polyunsaturated fatty acids in COVID-19: Rationale for the COVID-Omega-F trial. Frontiers in Physiology., 11, 624657. https://doi.org/10.3389/fphys.2020.624657
Ledford, H. (2020). Coronavirus breakthrough: Dexamethasone is first drug shown to save lives. Nature, 582(7813), 469. https://doi.org/10.1038/d41586-020-01824-5
Pyrillou, K., Chairakaki, A. D., Tamvakopoulos, C., & Andreakos, E. (2018). Dexamethasone induces omega3-derived immunoresolvents driving resolution of allergic airway inflammation. Journal of Allergy and Clinical Immunology. https://doi.org/10.1016/j.jaci.2018.04.004
Das, I., Png, C. W., Oancea, I., Hasnain, S. Z., Lourie, R., Proctor, M., et al. (2013). Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. Journal of Experimental Medicine., 210(6), 1201–1216. https://doi.org/10.1084/jem.20121268
Raut, A., & Huy, N. T. (2021). Rising incidence of mucormycosis in patients with COVID-19: Another challenge for India amidst the second wave? The Lancet Respiratory Medicine. https://doi.org/10.1016/S2213-2600(21)00265-4
FitzGerald, G. A. (2020). Misguided drug advice for COVID-19. Science, 367(6485), 1434. https://doi.org/10.1126/science.abb8034
Serhan, C. N., Chiang, N., & Van Dyke, T. E. (2008). Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology, 8(5), 349–361. https://doi.org/10.1038/nri2294
Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K., & Serhan, C. N. (2001). Lipid mediator class switching during acute inflammation: Signals in resolution. Nature Immunology., 2(7), 612–619. https://doi.org/10.1038/89759
Serhan, C. N., Clish, C. B., Brannon, J., Colgan, S. P., Chiang, N., & Gronert, K. (2000). Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. Journal of Experimental Medicine, 192(8), 1197–1204. https://doi.org/10.1084/jem.192.8.1197
Serhan, C. N., Hong, S., Gronert, K., Colgan, S. P., Devchand, P. R., Mirick, G., et al. (2002). Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. Journal of Experimental Medicine, 196(8), 1025–1037. https://doi.org/10.1084/jem.20020760
Hong, S., Gronert, K., Devchand, P. R., Moussignac, R. L., & Serhan, C. N. (2003). Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. Journal of Biological Chemistry., 278(17), 14677–14687. https://doi.org/10.1074/jbc.M300218200
Yousefifard, M., Zali, A., Zarghi, A., Madani Neishaboori, A., Hosseini, M., & Safari, S. (2020). Non-steroidal anti-inflammatory drugs in management of COVID-19; A systematic review on current evidence. International Journal of Clinical Practice, 74(9), e13557. https://doi.org/10.1111/ijcp.13557
Funding
No funding was received for this article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving Human Participants and/or Animals
No, this research uses the secondary data.
Informed Consent
Not applicable, this review uses the secondary data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahman, M.S., Hossain, M.S. Eicosanoids Signals in SARS-CoV-2 Infection: A Foe or Friend. Mol Biotechnol (2023). https://doi.org/10.1007/s12033-023-00919-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12033-023-00919-4