Abstract
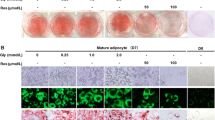
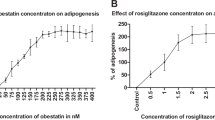
We have previously shown that cultured adipocytes have the ability to biosynthesize prostaglandin (PG) I2 called alternatively as prostacyclin during the maturation phase by the positive regulation of gene expression of PGI synthase and the prostanoid IP receptor. To clarify how prostacyclin regulates adipogenesis, we investigated the effects of prostacyclin and the specific agonists or antagonists for the IP receptor on the storage of fats during the maturation phase of cultured adipocytes. Exogenous PGI2 and the related selective agonists for the IP receptor including MRE-269 and treprostinil rescued the storage of fats attenuated by aspirin, a cyclooxygenase inhibitor. On the other hand, selective antagonists for IP such as CAY10441 and CAY10449 were effective to suppress the accumulation of fats as GW9662, a specific antagonist for peroxisome proliferator-activated receptor (PPAR)γ. Thus, pro-adipogenic action of prostacyclin can be explained by the action mediated through the IP receptor expressed at the maturation stage of adipocytes. Cultured adipocytes incubated with each of PGI2 and MRE-269 together with troglitazone, an activator for PPARγ, exhibited additively higher stimulation of fats storage than with either compound alone. The combined effect of MRE-269 and troglitazone was almost abolished by co-incubation with GW9662, but not with CAY10441. Increasing concentrations of troglitazone were found to reverse the inhibitory effect of CAY10441 in a dose-dependent manner while those of MRE-269 failed to rescue adipogenesis suppressed by GW9662, indicating the critical role of the PPARγ activation as a downstream factor for the stimulated adipogenesis through the IP receptor. Treatment of cultured adipocytes with cell permeable stable cAMP analogues or forskolin as a cAMP elevating agent partly restored the inhibitory effect of aspirin. However, excess levels of cAMP stimulated by forskolin attenuated adipogenesis. Supplementation with H-89, a cell permeable inhibitor for protein kinase A (PKA), had no effect on the promoting action of PGI2 or MRE-269 along with aspirin on the storage of fats, suggesting that the promotion of adipogenesis mediated by the IP receptor does not require the PKA activity.









Similar content being viewed by others
Abbreviations
- PG:
-
Prostaglandin
- PPAR:
-
Peroxisome proliferator-activated receptor
- PKA:
-
Protein kinase A
- C/EBP:
-
CCAAT enhancer-binding protein
- 15d-PGJ2 :
-
15-Deoxy-Δ12,14-PGJ2
- IBMX:
-
3-Isobutyl-1-methylxanthine
- COX:
-
Cyclooxygenase
- DMEM-HEPES:
-
Dulbecco’s modified Eagle medium with 25 mM HEPES
- FBS:
-
Fetal bovine serum
- 8-CPT-cAMP:
-
8-(4-Chlorophenyl)thio-cAMP
- 8-CPT-2′-O-Me-cAMP:
-
8-(4-Chlorophenyl)thio-2′-O-methyl-cAMP
- GM:
-
Growth medium
- DM:
-
Differentiation medium
- MM:
-
Maturation medium
- PBS (−):
-
Phosphate-buffered saline without Ca2+ and Mg2+
References
Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW (2001) IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor γ1. Proc Natl Acad Sci USA 98:2443–2448
Catalioto RM, Gaillard D, Maclouf J, Ailhaud G, Négrel R (1991) Autocrine control of adipose cell differentiation by prostacyclin and PGF2α. Biochim Biophys Acta 1091:364–369
Chawla A, Schwartz EJ, Dimaculangan DD, Lazar MA (1994) Peroxisome proliferator-activated receptor (PPAR)γ: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135:798–800
Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402
Chu X, Xu L, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K (2009) Suppression of adipogenesis program in cultured preadipocytes transfected stably with cyclooxygenase isoforms. Biochim Biophys Acta 1791:273–280
Clark RD, Jahangir A, Severance D, Salazar R, Chang T, Chang D, Jett MF, Smith S, Bley K (2004) Discovery and SAR development of 2-(phenylamino) imidazolines as prostacyclin receptor antagonists. Bioorg Med Chem Lett 14:1053–1056
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105
Falcetti E, Flavell DM, Staels B, Tinker A, Haworth SG, Clapp LH (2007) IP receptor-dependent activation of PPARγ by stable prostacyclin analogues. Biochem Biophys Res Commun 360:821–827
Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM (1995) 15-Deoxy-Δ12,14-PGJ2 is a ligand for the adipocyte determination factor PPARγ. Cell 83:803–812
Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA 94:4312–4317
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
Green H, Kehinde O (1974) Sublines of mouse 3T3 cells that accumulate lipid. Cell 1:113–116
Green H, Kehinde O (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5:19–27
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78:783–809
Ham JK, Park BH, Farmer SR (2001) A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem 276:18464–18471
Hansen JB, Zhang H, Rasmussen TH, Petersen RK, Flindt EN, Kristiansen K (2001) Peroxisome proliferator-activated receptor δ (PPARδ)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J Biol Chem 276:3175–3182
Hossain MS, Chowdhury AA, Rahman MS, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K (2011) Development of enzyme-linked immunosorbent assay for ∆12-prostaglandin J2 and its application to the measurement of the endogenous product generated by cultured adipocytes during the maturation phase. Prostaglandins Other Lipid Mediat 94:73–80
Hyman BT, Stoll LL, Spector AA (1982) Prostaglandin production by 3T3-L1 cells in culture. Biochim Biophys Acta 713:375–385
Insel PA, Ostrom RS (2003) Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol 23:305–314
Kliewer SA, Lenhard JM, Wilson TM, Patel I, Morris DC, Lehmann JM (1995) A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell 83:813–819
Kuri-Harcuch W, Green H (1978) Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci USA 75:6107–6109
Kuwano K, Hashino A, Asaki T, Hamamoto T, Yamada T, Okubo K, Kuwabara K (2007) 2-[4-[(5,6-Diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetamide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther 322:1181–1188
Lim H, Dey SK (2002) Minireview: a novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology 143:3207–3210
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu S, Nishimura K, Hossain MA, Jisaka M, Nagaya T, Yokota K (2004) Regulation and role of arachidonate cascade during changes in life cycle of adipocytes. Appl Biochem Biotechnol 118:133–153
Markwell MA, Hass SM, Toleret NE, Bieber LI (1981) Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol 72:296–303
Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, Guesnet P, Amri EZ, Négrel R, Ailhaud G (2003) Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res 44:271–279
Mazid MA, Chowdhury AA, Nagao K, Nishimura K, Jisaka M, Nagaya T, Yokota K (2006) Endogenous 15-deoxy-Δ12,14-PGJ2 synthesized by adipocytes during maturation phase contributes to upregulation of fat storage. FEBS Lett 580:6885–6890
Mei FC, Qiao J, Tsygankova QM, Meinkoth JL, Quilliam LA, Chen X (2002) Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277:11497–11504
Narumiya S, Sugimoto Y, Ushikubi F (1999) Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226
Négrel R, Gaillard D, Ailhaud G (1989) Prostacyclin as a potent effector of adipose-cell differentiation. Biochem J 257:399–405
Olschewski H, Rose F, Schermuly R, Ghofrani HA, Enke B, Olschewski A, Seeger W (2004) Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacol Ther 102:139–153
Petersen RK, JØrgensen C, Rustan AC, FrØyland L, Muller-Decker K, Furstenberger G, Berge RK, Kristiansen K, Madsen L (2003) Arachidonic acid-dependent inhibition of adipocyte differentiation requires PKA activity and is associated with sustained expression of cyclooxygenases. J Lipid Res 44:2320–2330
Rahman MS, Khan F, Syeda PK, Nishimura K, Jisaka M, Nagaya T, Shono F, Yokota K (2014) Endogenous synthesis of prostacyclin was positively regulated during the maturation phase of cultured adipocytes. Cytotechnology 66:635–646
Sandberg M, Butt E, Nolte C, Fischer L, Halbrügge M, Beltman J, Jahnsen T, Genieser HG, Jastorff B, Walter U (1991) Characterization of Sp-5,6-dichloro-1-β-d-ribofuranosylbenzimidazole- 3′,5′-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J 279:521–527
Steinberg RA, Agard DA (1981) Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem 256:10731–10734
Tsuboi H, Sugimoto Y, Kainoh T, Ichikawa A (2004) Prostanoid EP4 receptor is involved in suppression of 3T3-L1 adipocyte differentiation. Biochem Biophys Res Commun 322:1066–1072
Whittle BJ, Moncada S, Whiting F, Vane JR (1980) Carbacyclin—a potent stable prostacyclin analogue for the inhibition of platelet aggregation. Prostaglandins 19:605–627
Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43:528–550
Wise H (2003) Multiple signalling options for prostacyclin. Acta Pharmacol Sin 24:625–630
Xu L, Nishimura K, Jisaka M, Nagaya T, Yokota K (2006) Gene expression of arachidonate cyclooxygenase pathway leading to the delayed synthesis of prostaglandins E2 and F2α in response to phorbol 12-myristate 13-acetate and action of these prostanoids during life cycle of adipocytes. Biochim Biophys Acta 1761:434–444
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers 25450128, 25-03091, and 26-04092.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Khan, F., Syeda, P.K., Nartey, M.N.N. et al. Stimulation of fat storage by prostacyclin and selective agonists of prostanoid IP receptor during the maturation phase of cultured adipocytes. Cytotechnology 68, 2417–2429 (2016). https://doi.org/10.1007/s10616-016-9960-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-016-9960-7