Abstract
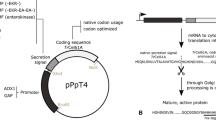
The sequences encoding the genes for endoglucanase II and cellobiohydrolase II from the fungus Trichoderma reesei QM9414 were successfully cloned and expressed in Yarrowia lipolytica under the control of the POX2 or TEF promoters, and using either the native or preproLip2 secretion signals. The expression level of both recombinant enzymes was compared with that obtained using Pichia pastoris, under the control of the AOX1 promoter to evaluate the utility of Y. lipolytica as a host strain for recombinant EGII and CBHII production. Extracellular endoglucanase activity was similar between TEF-preoproLip2-eglII expressed in Y. lipolytica and P. pastoris induced by 0.5 % (v/v) methanol, but when recombinant protein expression in P. pastoris was induced with 3 % (v/v) methanol, the activity was increased by about sevenfold. In contrast, the expression level of cellobiohydrolase from the TEF-preproLip2-cbhII cassette was higher in Y. lipolytica than in P. pastoris. Transformed Y. lipolytica produced up to 15 mg/l endoglucanase and 50 mg/l cellobiohydrolase, with the specific activity of both proteins being greater than their homologs produced by P. pastoris. Partial characterization of recombinant endoglucanase II and cellobiohydrolase II expressed in both yeasts revealed their optimum pH and temperature, and their pH and temperature stabilities were identical and hyperglycosylation had little effect on their enzymatic activity and properties.






Similar content being viewed by others
References
Boer, H., Teeri, T. T., & Koivula, A. (2000). Characterization of Trichoderma reesei cellobiohydrolase Cel7A secreted from Pichia pastoris using two different promoters. Biotechnology and Bioengineering, 69, 486–494.
Bordes, F., Fudalej, F., Dossat, V., Nicaud, J. M., & Marty, A. (2007). A new recombinant protein system for high-throughput screening in the yeast Yarrowia lipolytica. Journal of Microbiological Methods, 70, 493–502.
Cavallius, J., Zoll, W., Chakraburtty, K., & Merrick, W. C. (1993). Characterization of yeast EF-1α: Non-conservation of post-translational modifications. Biochimica et Biophysica Acta, 1163, 75–80.
Choi, N. S., Kim, B. H., Park, C. S., Han, Y. J., Lee, H. W., Choi, J. H., et al. (2009). Multiple-layer substrate zymography for detection of several enzymes in a single sodium dodecyl sulfate gel. Analytical Biochemistry, 386, 121–122.
Cregg, J. M., Cereghino, J. L., Shi, J., & Higgins, D. R. (2000). Recombinant protein expression in Pichia pastoris. Molecular Biotechnology, 16, 23–52.
Dashtban, M., Schraft, H., & Qin, W. (2009). Fungal bioconversion of lignocellulosic residues; opportunities and perspectives. International Journal of Biological Sciences, 5(6), 578–595.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59, 257–268.
Goyal, A., Ghosh, B., & Eveleigh, D. (1991). Characteristics of fungal cellulases. Bioresource Technology, 36, 37–50.
Ilmén, M., den Haan, R., Brevnova, E., McBride, J., Wiswall, E., Froehlich, A., et al. (2011). High level secretion of cellobiohydrolases by Saccharomyces cerevisiae. Biotechnology for Biofuels, 4, 30.
Ito, J., Fujita, Y., Ueda, M., Fukuda, H., & Kondo, A. (2004). Improvement of cellulose-degrading ability of a yeast strain displaying Trichoderma reesei endoglucanase II by recombination of cellulose-binding domains. Biotechnology Progress, 20, 688–691.
Kipper, K., Väljamäe, P., & Johansson, G. (2005). Processive action of cellobiohydrolase Cel7A from Trichoderma reesei is revealed as ‘burst’ kinetics on fluorescent polymeric model substrates. Biochemical Journal, 385, 527–535.
Kubicek, C. P. (1992). The cellulase proteins of Trichoderma reesei: Structure, multiplicity, mode of action and regulation of formation. Advances in Biochemical Engineering/Biotechnology, 45, 1–27.
Le Dall, M. T., Nicaud, J. M., & Gaillardin, C. (1994). Multiple-copy integration in the yeast Yarrowia lipolytica. Current Genetics, 26, 38–44.
Madzak, C., Gaillardin, C., & Beckerich, J. M. (2004). Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: A review. Journal of Biotechnology, 109, 63–81.
Mandels, M., & Weber, J. (1969). The production of cellulase. In: Cellulases and their applications. Advances in Chemistry Series, 95, 391–413.
Maris, A. J. A., Abbott, D. A., Bellissimi, E., Brink, J., Kuyper, M., Luttik, M. A. H., et al. (2006). Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: Current status. Antonie van Leeuwenhoek, 90, 391–418.
Masárová, J., Mislovicová, D., Gemeiner, P., & Michalková, E. (2001). Stability enhancement of Escherichia coli penicillin G acylase by glycosylation with yeast mannan. Biotechnology and Applied Biochemistry, 34, 127–133.
Messner, R., Kubicek-Pranz, E. M., Gsur, A., & Kubicek, C. P. (1991). Cellobiohydrolase II is the main conidial-bound cellulase in Trichoderma reesei and other Trichoderma strains. Archives of Microbiology, 155, 601–606.
Nakazawa, H., Okada, K., Kobayashi, R., Kubota, T., Onodera, T., Ochiai, N., et al. (2008). Characterization of the catalytic domains of Trichoderma reesei endoglucanase I, II, III, expressed in Escherichia coli. Applied Microbiology and Biotechnology, 81, 681–689.
Nelson, N. (1944). A photometric adaptation of the Somogyi method for determination of glucose. Journal of Biological Chemistry, 153, 375–380.
Nicaud, J. M., Fabre, E., & Gaillardin, C. (1989). Expression of invertase activity in Yarrowia lipolytica and its use as a selective marker. Current Genetics, 16(4), 253–260.
Nicaud, J. M., Madzak, C., Broek, P., Gysler, C., Duboc, P., Niederberger, P., et al. (2002). Protein expression and secretion in the yeast Yarrowia lipolytica. FEMS Yeast Research, 2, 371–379.
Okada, H., Sekiya, T., Yokoyama, K., Tohda, H., Kumagai, H., & Morikawa, Y. (1998). Efficient secretion of Trichoderma reesei cellobiohydrolase II in Schizosaccharomyces pombe and characterization of its products. Applied Microbiology and Biotechnology, 49, 301–308.
Park, C. S., Chang, C. C., & Ryu, D. D. Y. (2000). Expression and high-level secretion of Trichoderma reesei endoglucanase I in Yarrowia lipolytica. Applied Biochemistry and Biotechnology, 87, 1–15.
Penttilä, M. E., André, L., Lehtovaara, P., Bailey, M., Teeri, T. T., & Knowles, J. K. (1988). Efficient secretion of two fungal cellobiohydrolases by Saccharomyces cerevisiae. Gene, 63(1), 103–112.
Penttila, M. E., André, L., Saloheimo, M., Lehtovaara, P., & Knowles, J. K. (1987). Expression of two Trichoderma reesei endoglucanase in the yeast Saccharomyces cerevisiae. Yeast, 3, 175–185.
Poza, M., Sestelo, A. B. F., Ageitos, J. M., Vallejo, J. A., Veiga-Crespo, P., & Villa, T. G. (2007). Cloning and expression of the XPR2 gene from Yarrowia lipolytica in Pichia pastoris. Journal of Agricultural and Food Chemistry, 55, 3944–3948.
Qin, Y., Wei, X., Liu, X., Wang, T., & Qu, Y. (2008). Purification and characterization of recombinant endoglucanase of Trichoderma reesei expressed in Saccharomyces cerevisiae with higher glycosylation and stability. Protein Expression and Purification, 58, 162–167.
Saloheimo, M., Lehtovaara, P., Penttilä, M., Teeri, T. T., Ståhlberg, J., Johansson, G., et al. (1988). EGIII, a new endoglucanase from Trichoderma reesei: The characterization of both gene and enzyme. Gene, 63(1), 11–22.
Sambrook, J., Fritch, E. F., & Maniatis, T. (1989). Molecular cloning a laboratory manual (2nd ed., Vol. 1, 2 and 3). New York: Cold Spring Harbor Laboratory Press.
Schuster, A., & Schmoll, M. (2010). Biology and biotechnology of Trichoderma. Applied Microbiology and Biotechnology, 87, 787–799.
Somogyi, M. (1952). Note on sugar determination. Journal of Biological Chemistry, 195, 19–25.
Suominen, P. L., Mäntylä, A. L., Karhunen, T., Hakola, S., & Nevalainen, H. (1993). High frequency one-step gene replacement in Trichoderma reesei. II. Effect of deletions of individual cellulase gene. Molecular and General Genetics, 241, 523–530.
Teeri, T. T., Lehtovaara, P., Kauppinen, S., Salovuori, I., & Knowles, J. (1987). Homologous domains in Trichoderma reesei cellulolytic enzymes: Gene sequence and expression of cellobiohydrolase II. Gene, 51(1), 43–52.
van Zyl, W. H., Lynd, L. R., den Haan, R., & McBride, J. E. (2007). Consolidated bioprocessing for bioethanol production using Saccharomyces cerevisiae. Advances in Biochemical Engineering/Biotechnology, 108, 205–235.
Wood, T. M. (1988). Preparation of crystalline, amorphous, and dyed cellulase substrates. Methods in Enzymology, 160, 19–25.
Wu, S., & Letchworth, G. J. (2004). High efficiency transformation by electroporation of Pichia pastoris pretreated with lithium acetate and dithiothreitol. BioTechniques, 36, 152–154.
Zhang, Y. H. P., Himmel, M. E., & Mielenz, J. (2006). Outlook for cellulase improvement: Screening and selection strategies. Biotechnology Advances, 24, 452–481.
Acknowledgments
This work was financially supported by grants from Chulalongkorn University Dutsadi Phiphat Scholarship, Chulalongkorn University Graduate Scholarship to Commemorate the 72nd Anniversary of His Majesty King Bhumibol Adulyadej, and Mrs. Ratanavalee In-ochanon from the PTT Public Company Limited. The authors thank Florence Bordes and Alain Marty for precious help with using the Yarrowia expression system and Jean-Marc Nicaud for readily supplying plasmids and Yarrowia strains.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonvitthya, N., Bozonnet, S., Burapatana, V. et al. Comparison of the Heterologous Expression of Trichoderma reesei Endoglucanase II and Cellobiohydrolase II in the Yeasts Pichia pastoris and Yarrowia lipolytica . Mol Biotechnol 54, 158–169 (2013). https://doi.org/10.1007/s12033-012-9557-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9557-0