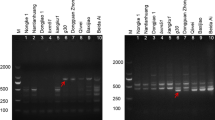
Abstract
Jatropha curcas L., a multipurpose shrub, has acquired significant economic importance for its seed oil which can be converted to biodiesel an emerging alternative to petro-diesel. In addition to the commercial value, it is also having medicinal and even high nutritional value to use as animal fodder which is limited due to the toxicity. Development of molecular marker will enable to differentiate non-toxic from toxic variety of J. curcas in a mixed population and also for quality control since the toxic components of J. curcas has deleterious effect on animals. In the present study, the efforts were made to generate the specific SCAR marker for toxic and/or non-toxic J. curcas from RAPD markers. Among the markers specific for toxic and non-toxic varieties, four were selected, purified, cloned, sequenced, and designed primers out of which one set of primers NT-JC/SCAR I/OPQ15-F and R could able to discriminate the non-toxic with toxic Jatropha by giving expected 430 bp size amplification in non-toxic variety. Furthermore, novel multiplex PCR was designed using the nrDNA ITS primers to overcome the false negatives. Present work also demonstrates utility of the conserved regions of nrDNA coding genes in ruling out the artifacts in PCR-like false negatives frequently occur in SCAR due to various reasons. The specific SCAR markers generated in the present investigation will help to distinguish non-toxic from toxic varieties of J. curcas or vice versa, and isolated marker along with designed multiplex protocol has applications in quality control for selective cultivation of non-toxic variety and will also assist in breeding and molecular mapping studies.




Similar content being viewed by others
References
Ghosh, A., Chaudhary, D. R., Reddy, M. P., Rao, S. N., Chikara, J., Pandya, J. B., et al. (2007). Prospects for Jatropha methyl ester (biodiesel) in India. International Journal of Environmental Studies, 64, 659–674.
Takeda, Y. (1982). Development study on Jatropha curcas (Sabudum) oil as a substitute for diesel engine oil in Thailand. Journal of Agricultural Association of China, 120, 1–8.
Mandpe, S., Kadlaskar, S., Degen, W., & Keppeler, S. (2005). On road testing of advanced common rail diesel vehicles with biodiesel from the Jatropha curcas plant (vol. 26, pp. 356–364). Society of Automotive Engineering Inc.
Makkar, H. P. S., & Becker, K. (1997). Potential of Jatropha seed cake as protein supplement in livestock feed and constraints to its utilization. In Proceedings of Jatropha 97: International symposium on biofuel and industrial products from Jatropha curcas and other tropical oilseed plants, Managua/Nicaragua, Mexico (pp. 23–27).
Makkar, H. P. S., Aderibigbe, A. O., & Becker, K. (1998). Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chemistry, 62, 207–215.
Martinez-Herrera, J., Sibdhiraju, S., Francis, G., Davila-Ortiz, G., & Becker, K. (2006). Chemical composition, toxic/antimetabolic constituents and effects of different treatments on their levels, in four provenances of Jatropha curcas L. from Mexico. Food Chemistry, 96, 80–89.
Ginwal, H. S., Rawat, P. S., & Srivastava, R. L. (2004). Seed source variation in growth performance and oil yield of Jatropha curcas Linn in central India. Silve Senetica, 53, 186–192.
Sujatha, M., Makkar, H. P. S., & Becker, K. (2005). Shoot bud proliferation from axillary nodes and leaf sections of non-toxic Jatropha curcas L. Plant Growth Regulations, 47, 83–90.
Francis, G., Edingger, R., & Becker, K. (2005). A concept for simultaneous wasteland reclamation fuel production and socio economic development in degraded areas in India need potential & perspectives of Jatropha plantation. Natural Resources Forum, 29, 12–24.
Becker, K., & Makkar, H. P. S. (1998). Effects of phorbol esters in carp (Cyprinus carpio L.). Veterinary Human Toxicology, 40, 82–89.
Sudheer, P. D. V. N., Singh, S., Mastan, S. G., Patel, J., & Reddy, M. P. (2009). Molecular characterization and identification of markers for toxic and non-toxic varieties of Jatropha curcas L. using RAPD, AFLP and SSR markers. Molecular Biology Reports, 36(6), 1357–1364.
Sudheer, P. D. V. N., Sarkar, R., Meenakshi, Boricha, G., & Reddy, M. P. (2009). A simple protocol for isolation of high quality genomic DNA from Jatropha curcas for genetic diversity and molecular marker studies. Indian Journal of Biotechnology, 8, 187–192.
Williams, J. G., Kubelik, A. R., Livak, J., Rafalski, J., & Tingey, S. V. (1990). DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Resources, 18, 6531–6535.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Sudheer, P. D. V. N., Nirali, P., Reddy, M. P., & Radhakrishnan, T. (2009). Comparative study of interspecific genetic divergence and phylogenic analysis of genus Jatropha by RAPD and AFLP. Molecular Biology Reports, 36, 901–907.
Jones, N., & Miller, J. H. 1991. Jatropha curcas a multipurpose species for problematic sites. Land and Resources Series No. 1 (pp. 1–12). Washington: Asia Technical Department, World Bank.
Heller, J. (1996). Physic nut-Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops. Rome: International Plant Genetic Resources Institute.
Gubitz, G. M., Mittelbach, M., & Trabi, M. (1999). Exploitation of the tropical oil seed plant Jatropha curcas L. Bioresources Technology, 67, 73–82.
Makkar, H. P. S., Becker, K., Sporer, F., & Wink, M. (1997). Studies on nutritive potential and toxic constituents of different provenance of Jatropha curcas. Journal of Agricultural Food Chemistry, 45, 3152–3157.
Makkar, H. P. S., Becker, K., & Schmoo, B. (1998). Edible provenances of Jatropha curcas from Quinatana Roo state of Mexico and effect of roasting antinutrient and toxic factors in seeds. Plant Foods for Human Nutrition, 52, 31–36.
Sudheer, P. D. V. N., Balaji, C., & Reddy, M. P. (2009). Genetic diversity and phylogenetic analysis of genus Jatropha based on nrDNA ITS sequence. Molecular Biology Reports, 36, 1929–1935.
Acknowledgments
We are thankful to Council for Scientific and Industrial Research, New Delhi, India, for its financial support and for research associate fellowship. We would like to thank Professor B. N. Tiwari, Head, School of biotechnology, for his kind support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mastan, S.G., Sudheer, P.D.V.N., Rahman, H. et al. Development of SCAR Marker Specific to Non-Toxic Jatropha curcas L. and Designing a Novel Multiplexing PCR Along with nrDNA ITS Primers to Circumvent the False Negative Detection. Mol Biotechnol 50, 57–61 (2012). https://doi.org/10.1007/s12033-011-9415-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9415-5