Abstract
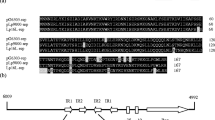
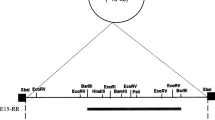
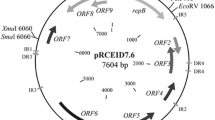
A cryptic plasmid pD403 was isolated from Lactobacillus plantarum D403 derived from fermented dairy products. It was 2,791 bp in size with a G+C content of 37%. Nucleotide sequence analysis revealed two open reading frames, orf1 and orf2. ORF1 (318 amino acids) was identified as a replication protein (RepA). ORF2 (137 amino acids) shared 31% similarity with the transcriptional regulator of Ralstonia pickettii 12D. Functional investigation indicated that ORF2 (Tra) had the ability of improving the transformation efficiency. The origin of replication was predicted, suggesting that pD403 was a rolling-circle-replication (RCR) plasmid. An Escherichia coli/Lactobacillus shuttle vector pCD4032 was constructed based on the pD403 replicon, and proved to be successfully transformed into various lactobacilli including Lactobacillus casei, Lactobacillus plantarum, Lactobacillus fermentum, and Lactobacillus brevis. The transformation efficiencies were ranged from 1.3 × 102 to 7 × 104 transformants per microgram DNA. Furthermore, an expression vector pCD4033 was developed with the promoter of the lactate dehydrogenase from Lactobacillus delbrueckii 11842. The green fluorescent protein (gfp) as a reporter was expressed successfully in various lactobacilli tested, suggesting that the expression vector pCD4033 had the potential to be used as a molecular tool for heterologous gene cloning and expression in lactobacilli.





Similar content being viewed by others
References
Marteau, P., Seksik, P., Lepage, P., & Doré, J. (2004). Cellular and physiological effects of probiotics and prebiotics. Mini Reviews in Medicinal Chemistry, 4, 889–896.
Giorgi, P. L. (2009). Probiotics. A review. Recenti Progressi in Medicina, 100, 40–47.
Seegers, J. F. (2002). Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends in Biotechnology, 20, 508–515.
Vaughan, E. E., Schut, F., Heilig, H. G., Zoetendal, E. G., de Vos, W. M., & Akkermans, A. D. (2000). A molecular view of the intestinal ecosystem. Current Issues in Intestinal Microbiology, 1, 1–12.
Mathiesen, G., Sørvig, E., Blatny, J., Naterstad, K., Axelsson, L., & Eijsink, V. G. (2004). High-level gene expression in Lactobacillus plantarum using a pheromone-regulated bacteriocin promoter. Letters in Applied Microbiology, 39, 137–143.
Sørvig, E., Mathiesen, G., Naterstad, K., Eijsink, V. G., & Axelsson, L. (2005). High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology, 151, 2439–2449.
Desmond, C., Ross, R. P., Fitzgerald, G., & Stanton, C. (2005). Sequence analysis of the plasmid genome of the probiotic strain Lactobacillus paracasei NFBC338 which includes the plasmids pCD01 and pCD02. Plasmid, 54, 160–175.
An, H. Y., & Miyamoto, T. (2006). Cloning and sequencing of plasmid pLC494 isolated from human intestinal Lactobacillus casei: Construction of an Escherichia coli-Lactobacillus shuttle vector. Plasmid, 55, 128–134.
Crutz-Le Coq, A. M., & Zagorec, M. (2008). Vectors for Lactobacilli and other Gram-positive bacteria based on the minimal replicon of pRV500 from Lactobacillus sakei. Plasmid, 60, 212–220.
Lee, J. H., Halgerson, J. S., Kim, J. H., & O’Sullivan, D. J. (2007). Comparative sequence analysis of plasmids from Lactobacillus delbrueckii and construction of a shuttle cloning vector. Applied and Environmental Microbiology, 73, 4417–4424.
Yin, S., Hao, Y., Zhai, Z., Li, R., Huang, Y., Tian, H., et al. (2008). Characterization of a cryptic plasmid pM4 from Lactobacillus plantarum M4. FEMS Microbiology Letters, 285, 183–187.
Alvarez-Martín, P., O’Connell-Motherway, M., van Sinderen, D., & Mayo, B. (2007). Functional analysis of the pBC1 replicon from Bifidobacterium catenulatum L48. Applied Microbiology and Biotechnology, 76, 1395–1402.
Oozeer, R., Goupil-Feuillerat, N., Alpert, C. A., van de Guchte, M., Anba, J., Mengaud, J., et al. (2002). Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human flora-associated mice. Applied and Environmental Microbiology, 68, 3570–3574.
Neu, T., & Henrich, B. (2003). New thermosensitive delivery vector and its use to enable nisin-controlled gene expression in Lactobacillus gasseri. Applied and Environmental Microbiology, 69, 1377–1382.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
O’Sullivan, D. J., & Klaenhammer, T. R. (1993). Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Applied and Environmental Microbiology, 59, 2730–2733.
Bringel, F., & Hubert, J.-C. (1990). Optimized transformation by electroporation of Lactobacillus plantarum strains with plasmid vectors. Applied Microbiology and Biotechnology, 33, 664–670.
Pérez-Arellano, I., & Pérez-Martínez, G. (2003). Optimization of the green fluorescent protein (GFP) expression from a lactose-inducible promoter in Lactobacillus casei. FEMS Microbiology Letters, 222, 123–127.
Kaneko, Y., Kobayashi, H., Kiatpapan, P., Nishimoto, T., Napitupulu, R., Ono, H., et al. (2000). Development of a host-vector system for Lactobacillus plantarum L137 isolated from a traditional fermented food produced in the Philippines. Journal of Bioscience and Bioengineering, 89, 62–67.
Sørvig, E., Skaugen, M., Naterstad, K., Eijsink, V. G., & Axelsson, L. (2005). Plasmid p256 from Lactobacillus plantarum represents a new type of replicon in lactic acid bacteria, and contains a toxin-antitoxin-like plasmid maintenance system. Microbiology, 151, 421–431.
Khan, S. A. (1997). Rolling-circle replication of bacterial plasmids. Microbiology and Molecular Biology Reviews, 61, 442–455.
Bouia, A., Bringel, F., Frey, L., Kammerer, B., Belarbi, A., Guyonvarch, A., et al. (1989). Structural organization of pLP1, a cryptic plasmid from Lactobacillus plantarum CCM 1904. Plasmid, 22, 185–192.
Hong, S. H. (1995). Enhancing survival of lactic acid bacteria in ice cream by natural encapsulation and gene transfer. Thesis, Food Science & Human Nutrition, University of Missouri, Columbia.
Leer, R. J., Van Luijk, N., Posno, M., & Pouwels, P. H. (1992). Structural and functional analysis of two cryptic plasmids from Lactobacillus pentosus MD353 and Lactobacillus plantarum ATCC 8014. Molecular and General Genetics, 234, 265–274.
Heng, N. C. K., Bateup, J. M., Loach, D. M., Wu, X., Jenkinson, H. F., Morrison, M., et al. (1999). Influence of different functional elements of plasmid pGT232 on maintenance of recombinant plasmids in Lactobacillus reuteri populations in vitro and in vivo. Applied and Environmental Microbiology, 65, 5378–5385.
Frère, J., Novel, M., & Novel, G. (1993). Molecular analysis of the Lactococcus lactis subspecies lactis CNRZ270 bidirectional theta replicating lactose plasmid pUCL22. Molecular Microbiology, 10, 1113–1124.
Gravesen, A., Josephsen, J., von Wright, A., & Vogensen, F. K. (1995). Characterization of the replicon from the lactococcal theta-replicating plasmid pJW563. Plasmid, 34, 105–118.
Hayes, F., Vos, P., Fitzgerald, G. F., de Vos, W. M., & Daly, C. (1991). Molecular organization of the minimal replicon of novel, narrow-host-range, lactococcal plasmid pCI305. Plasmid, 25, 16–26.
Sánchez, C., Hernández de Rojas, A., Martínez, B., Argüelles, M. E., Suárez, J. E., Rodríguez, A., et al. (2000). Nucleotide sequence and analysis of pBL1, a bacteriocin-producing plasmid from Lactococcus lactis IPLA 972. Plasmid, 44, 239–249.
Eynard, N., & Teissie, J. (2000). Electrotransformation of bacteria. Springer Lab Manual.
van de Guchte, M., Kodde, J., van der Vossen, J. M., Kok, J., & Venema, G. (1990). Heterologous gene expression in Lactococcus lactis subsp. lactis: synthesis, secretion, and processing of the Bacillus subtilis neutral protease. Applied and Environmental Microbiology, 56, 2606–2611.
Guimarães, V. D., Innocentin, S., Lefèvre, F., Azevedo, V., Wal, J. M., Langella, P., et al. (2006). Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells. Applied and Environmental Microbiology, 72, 7091–7097.
Stéphane, H., Laetitia, P., Vasco, A., Gérard, C., Jean-Michel, W., & Philippe, L. (2007). Efficient production and secretion of bovine β-lactoglobulin by Lactobacillus casei. Microbial Cell Factories, 6, 12.
Zhang, X., Kong, J., & Qu, Y. (2006). Isolation and characterization of a Lactobacillus fermentum temperate bacteriophage from Chinese yogurt. Journal of Applied Microbiology, 101, 857–863.
van de Guchte, M., Penaud, S., Grimaldi, C., Barbe, V., Bryson, K., Nicolas, P., et al. (2006). The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proceedings of the National Academy of Science, 103, 9274–9279.
Kuipers, O. P., de Ruyter, P. G. G. A., Kleerebezem, M., & de Vos, W. M. (1998). Quorum sensing-controlled gene expression in lactic acid bacteria. Journal of Biotechnology, 64, 15–21.
Mierau, I., & Kleerebezem, M. (2005). 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus. Applied Microbiology and Biotechnology, 68, 705–717.
Waldo, G. S., Standish, B. M., Berendzen, J., & Terwilliger, T. C. (1999). Rapid protein-folding assay using green fluorescent protein. Nature Biotechnology, 17, 691–695.
Acknowledgments
We are grateful to Prof. Jan-Willem de Gier for kindly providing the plasmid pWaldo-control. We also thank M. van de Guchte, Hazebrouck, and Dr. N. Galleron for their generous gifts of Lb. delbrueckii subsp. bulgaricus ATCC 11842, Lb. casei BL23, and L. lactis NZ9000, respectively. This research was supported by grants from 863 Hi-Tech Research and Development Program of China (grant no. 2006AA10Z321 and 2008AA10Z335).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Z., Kong, J. & Kong, W. Characterization of a Cryptic Plasmid pD403 from Lactobacillus plantarum and Construction of Shuttle Vectors Based on its Replicon. Mol Biotechnol 45, 24–33 (2010). https://doi.org/10.1007/s12033-010-9242-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-010-9242-0