Abstract
Background
pTE15 is a ~ 15-kb narrow-host-range indigenous plasmid from Lactobacillus reuteri N16 that does not replicate in selected Bacillus spp., Staphylococcus spp., and other Lactobacillus spp.
Methods
Combined deletion analysis the minireplicon essential of pTE15 with replicon-probe vector pUE80 (−) to confirmed sufficient for replication and from the ssDNA intermediate detection, plasmid amplification tested by chloramphenicol treatment, and replication origin sequence analysis to delineated the novel theta-type replication of pTE15.
Results
Single-stranded intermediate of pTE15 DNA was not detected in L. reuteri, indicating that this plasmid does not replicate via a rolling circle mechanism. The replicon of pTE15 did not display the structural organization typical of rolling-circle plasmids, nor were they similar to known rolling-circle plasmids. We further provided evidence that this plasmid applied a new mode of theta-type replication mechanism: (1) the size of this plasmid was > 10-kb; (2) the minireplicon consisted of AT-rich (directed repeat, iteron) and DnaA sequences; (3) the minireplicon did not contain double-strand origin (DSO) and essential rep genes, and it also showed no single-strand origin (SSO) structure; (4) the intermediate single-stranded DNA products were not observed for pTE15 replication; (5) the minireplicon did not contain a typical essential replication protein, Rep, (6) its copy number was decreased by chloramphenicol treatment, and (7) genes in pTE15 replication region encoded truncated RepA (TRepA), RepB and RepC, which were replication-associated proteins, but they were not essential for pTE15 replication.
Conclusions
Collectively, our results strongly suggested that the indigenous plasmid pTE15 of L. reuteri N16 belongs to a new class of theta replicons.
Similar content being viewed by others
Background
Probiotics are generally defined as “live microorganisms which, when administered in adequate amounts, can confer a health benefit on the host” [1]. Presently, various probiotics, including Lactobacillus spp., Bifidobacterium spp., Saccharomyces boulardii, Propionibacterium spp., Streptococcus spp., Bacillus spp., Enterococcus spp., and some specific Escherichia coli strains, have been found to confer diverse health benefits to the host [2]. Among these potential probiotics, L. reuteri, which was first isolated in 1962, has been recognized as an obligatory heterofermentative and endogenous lactic acid bacterial species in the GI tract of mammals [3]. Furthermore, specific L. reuteri strains have been found to display various beneficial effects, including the production of antimicrobial molecules such as organic acids, ethanol, and reuterin [4]. Several L. reuteri strains can decrease the levels of pro-inflammatory cytokines and thus promote the function and development of regulatory T cells [5]. In addition, specific L. reuteri strains have been shown to reduce the incidence and severity of diarrhea, prevent colic and necrotic enterocolitis, and maintain a functional mucosal barrier in humans [5,6,7]. Collectively, specific L. reuteri strains have been used to promote growth, improve the efficiency of feed utilization, prevent diarrhea, and enhance the immune system in several animals [5, 8].
As L. reuteri is an obligatory, heterofermentative, and endogenous probiotic species in the GI tract of mammals, some studies have attempted to develop it or other specific Lactobacillus species as vaccine carriers [9,10,11]. Notably, a vector with a narrow host range is considered an important factor, and this type of vector is less likely to be horizontally transferred between bacterial species [12]. Moreover, it is generally accepted that the replicon origins of a vector are important regions that influence their host range, and subtle point mutations in these regions have a considerable impact on the plasmid’s host range [13, 14]. Therefore, the isolation and characterization of a set of plasmids that can replicate simultaneously in Lactobacillus hosts would be of great advantage for the construction of genetically altered strains [15,16,17].
Interestingly, in our previous study, we first reported two specific L. reuteri strains L1 and N16, which were isolated from the small intestines of chickens, containing a 7.0-kb plasmid (pTE80) or ~ 15-kb plasmid (pTE15), respectively. The two plasmids encode genes conferring resistance to erythromycin (Em) and have a narrow host range. Therefore, we further constructed a replicon-probe vector to identify the replication regions (RR) from the indigenous plasmids of L. reuteri [18] and applied this replicon-probe vector to identify the replication characteristics that was not rely exclusively on host initiation factors for the RR of the pTE82 plasmid, which is also an indigenous plasmid of the poultry strain L. reuteri G4 [19]. We previously reported for the first time that this plasmid contained a palT-type single-strand origin (SSO) from Lactobacillus [19].
To date, the theta-type replication plasmid in lactobacilli were including pLP60 [20], pRC12 and pRC18 [21], pRV500 [22], pREN [23], and pLJ1 [24], especially the pRC12, pRC18, pRV500, and pREN belonged to the pUCL287 subfamily. They were class A of theta replicons that must plasmid encoded Rep protein with a related origin of replication and replicate independently of DNA pol I. Most lactic acid bacteria (LAB) theta-type replicating plasmids belong to this class [25]. Notably, there are six known classes of theta replicons [15,16,17, 26]. However, our data suggest that pTE15 cannot be classified into any one of the six known classes of theta replicons. To our knowledge, this is the first unclassified classes of theta replicons in indigenous plasmid of Lactobacillus to apply the theta replication machinery.
This study aimed to identify and characterize narrow-host-range plasmid, pTE15 of L. reuteri N16 to facilitate the development of L. reuteri as a vaccine carrier and pharmaceutical applications. Understanding plasmid maintenance and replication have significant practical implications for clinical studies and bioremediation. We found that pTE15 employed a theta-type replication mechanism, and its replication relied exclusively on host initiation factors.
Results
Replication region of pTE15
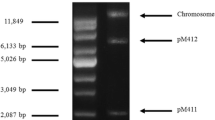
To determine the essential region of pTE15 required for replication, pTE15/19 was digested with BsrBI, and the obtained 6.8-kb fragment was subcloned into pUE80 (−) [18], which had been digested with HincII to obtain recombinant plasmid pTE15-RR, both pTE15-RR and pUE 80 (−) were further digested with XbaI/PstI and subcloned together to get 3.1-kb DNA fragment, named pTE15-RO. (Fig. 1 and Table 1), and they were further transformed into L. reuteri DSM 20016 by electroporation. The recombinant L. reuteri DSM 20016 can survive in a medium with Em, initially indicating that this plasmid contains the essential RR (6.8-kb and 3.1-kb fragments). Further, the identification of the RR of pTE15 was confirmed with pTE15-RO purified from recombinant E. coli and L. reuteri DSM 20016 then assessed by agarose gel electrophoresis, showing pTE15-RO contained the 3.1-kb DNA fragment could replicate in L. reuteri DSM 20016 (Fig. 2). Finally, the data supported that pTE15-RR and pTE15-RO contained the replicon because these derivatives helped the recombinant L. reuteri DSM 20016 grow well in MRS (De Man, Rogosa and Sharpe; Oxoid) medium with Em (10 μg/mL).
Identification of the replication region of pTE15 by DNA electrophoresis. The recombinant plasmids were undigested by restriction enzyme; lane M1: supercoiled DNA marker (Sigma-Aldrich), lanes 1, 2: pUE80 (−) (3.8-kb) and pTE15-RO (6.9-kb) were purified from E. coli TG1, lane 3: pTE15-RO was purified from L. reuteri 20016. The recombinant plasmids were digested by XbaI/PstI, lanes 4, 5: pUE80 (−) and pTE15-RO were purified from E. coli TG1, lane 6: pTE15-RO was purified from recombinant L. reuteri DSM 20016. lane M2: λDNA/HindIII marker
Next, we conducted sequence and functional analysis of the RR (3487-bp) of the L. reuteri plasmid pTE15, which was inferred from DNA sequence analysis (Fig. 3B). From the RR sequence of pTE15 was aligned with non-redundant sequences in NCBI, result shown the most similarity plasmid is pLRI04 and the nucleotides (1–1534-bp) at upstream of replication origin (directed repeat, iterons) of pTE15 has 95.8% identities with plasmid pLRI04 of L. reuteri I5007 isolated from health pig (Supplementary Fig. 1) (unpublished data).
DNA sequence of the 3487-bp fragment of pTE15 containing all the information required for autonomous replication. A Schematic representation of the pTE15 minireplicon contained IR, SDR, LDR (AT-rich, iteron), DnaA box, and cer-like site. B The putative amino acid sequences of RepA, RepB and RepC are presented below the DNA sequence. Possible − 35, − 10, and ribosome binding sites (RBS) are presented upper the lowercase DNA sequence; inverted repeated (IR) sequences are indicated with single-line arrows; possible iteron DNA sequences (TAGTRRR) of pAD1 are presented italic word. The following characteristics are indicated: =DnaA is a DnaA-like box; cer like site is a cer-like resolution site. Regions characterized by a high AT content and including direct repeats are bolded and shaded, except the CTTGCTCTCTC sequence. The following short ORFs, lacking a potential RBS at an appropriate distance from the putative start codon, are present upstream of the transcribed repB and repC: (i) lower strand, position 1029–901, MLMGQGQGAF-KLSALQLLMS-SAAVFAKLIN-DCDGLTERAM-FS*; (ii) lower strand, position 1127–1023, MMVSHSSNSL-ICCLLNKKNQ-PAEQGLGGNT-RACE*; (iii) upper strand, position 1149–1238, MGYLKHFQGF-LATLLVLCDK-HWDWNSIDC*; (iv) upper strand, position 1281–1442, MRLRCRATAS-EFTGCDPKEK-ACSRLATHFL-SSSGFNSSFD-NLNFSPFLSI-LYI*
For the DNA sequence analysis (Fig. 3B), the putative amino acid sequences of non-essential truncated RepA protein (TRepA) in the upstream of replication origin and RepB and RepC are presented below the replication origin. Possible − 35, − 10, and ribosome binding sites (RBS) are presented above the lowercase DNA sequence, which were predicted by blastx [29].
The 112 amino acid sequence of truncated RepA (TRepA) protein shared 99% identity with C-terminal residuals of Rep proteins of pRK11281 (Accession No. WP_016497314, unpublished) in Limosilactobacillus reuteri. RepB (295 aa) and RepC (123 aa) were also had 99% identities with AAA family ATPase (Accession No. WP_098035017) and replication-associated protein RepC (WP_098035018), respectively in Limosilactobacillus antri (unpublished), but these putative Rep proteins were not essential for pTE15 replication.
The replication origin had inverted repeat (IR) sequences are indicated with single-line arrows; directed repeat (DR) sequences are indicated with shadows and bold words; possible iteron DNA sequences (TAGTRRR) of pAD1 [30] are presented in italics. The following short ORFs, lacking a potential RBS at an appropriate distance from the putative start codon, are presented upstream of the transcribed repB and repC: (i) lower strand, position 1029–901, MLMGQGQGAF-KLSALQLLMS-SAAVFAKLIN-DCDGLTERAM-FS*; (ii) lower strand, position 1127–1023, MMVSHSSNSL-ICCLLNKKNQ-PAEQGLGGNT-RACE*; (iii) upper strand, position 1149–1238, MGYLKHFQGF-LATLLVLCDK-HWDWNSIDC*; and (iv) upper strand, position 1281–1442, MRLRCRATAS-EFTGCDPKEK-ACSRLATHFL-SSSGFNSSFD-NLNFSPFLSI-LYI*.
Nucleotide sequence accession number
Replication region (3487-bp) and the full-length sequence (15322-bp) the entire plasmid pTE15 were deposited into NCBI GenBank with accession number AF036766 and submission ID: 2637896.
Minireplicon of pTE15
The functional analysis of the RR of the L. reuteri plasmid pTE15, which was inferred from the DNA sequence analysis shown in Fig. 3B. As shown in Fig. 4, putative ORFs (black arrows), cis-acting regions (light gray box), and promoters (arrowheads) are shown on the linear restriction map.
Functional analysis of the replication region of the L. reuteri plasmid pTE15. A linear restriction map is shown at the bottom of the diagram. Putative ORFs (black arrows), cis-acting region (light grey box), and promoters (arrowheads) shown on the restriction map were inferred from sequence analysis. Think lines above the linear map represent the fragments cloned into pUE80 (−). The names of recombinant plasmids containing these inserts are indicated on the left. The replication phenotype (Rep) is indicated on the right. The vertical lines delimit the core replicon
To further determine and confirm the minireplicon of pTE15 required for replication, we constructed a series of recombinant plasmids with different deletions, including pTE15-RRΔ1 to pTE15-RRΔ7 (Table 1), and this set of recombinant plasmids was also compared and is shown in Fig. 4. The resulting plasmids were tested for their ability to replicate and maintain in recombinant L. reuteri DSM 20016 with Em selection. We observed that recombinant L. reuteri DSM 20016 with pTE15-RRΔ5 and pTE15-RRΔ6 could not grow in a medium with Em (10 μg/mL), and the other recombinant L. reuteri DSM 20016 survived in a medium with Em, indicating that the other recombinant plasmids contained the RR. Collectively, compared with the deletion set of recombinant plasmid derivatives, we confirmed that pTE15-RRΔ7 contains the essential minireplicon of pTE15 required replication. Comparison of pTE15-RRΔ4 and pTE15-RRΔ5 shown the sequence between HindIII and SacI cutting site, the three IR, DnaA box, and three short DR (SDR, 5′-TTGCTC-3′) (Fig. 3) were essential for pTE15 replication.
This replication origin in pTE15-RRΔ7 contained 1311-bp was genetic analysis by GCG Wisconsin Package (UWGCG) [29, 31] and DoriC 10.0 bioinformatics (http://tubic.org/doric/ and http://tubic.tju.edu.cn/doric/) [32] revealed that this region contained eight IR, three SDR and three AT-rich long DR (LDR) sequences (51-bp, interon), as well as six structures (=DnaA), three Fis binding motif, two DnaA-trio, two CtrA binding motif, and 2 GATC as shown in Fig. 3A. In addition, this region also displayed one structure (cer-like site) that was similar to the pColE1 cer sequence.
The potential site-specific recombination of pTE15 was also compared with that of the E. coli chromosome and plasmid, as shown in Fig. 5. A few identical sequences were recognized between the cer-like site of pTE15 (position 1624–1652 in Fig. 3B, 5’-GCTGTAGTACGTTATATATTGTGGTAAAT-3′) and the chromosome of E. coli (RecA-independent recombination site, dif), pColE1 (cer), and pSC101(psi) of E. coli. The highly identity of pTE15 cer-like site and pColE1 cer was 71.4% with gap-excluded, but a short DNA sequence of 12-bp within the minoreplicon (position 1641–1652) was 91.7% identity of them (Fig. 5).
Potential site-specific recombination site of pTE15. Alignment between the RecA-independent recombination sites dif of the E. coli chromosome, cer and psi of the E. coli plasmids pColE1 and pSC101 respectively and the homologous region of pTE15. Identical sequences are bolded and identical sequences of pTE15 and pColE1 cer are boxed.
Collectively, the above data support that pTE15 employ a theta form replication mechanism that could be a class B (pColE1 type) mode that was not necessary plasmid encoded Rep protein for ColE1 replication. Thus, we performed further experiments to dissect these issues, as explained below.
Absence of single-stranded DNA in cells harboring pTE15
Rolling-circle replication (RCR) plasmids are known to produce ssDNA replication intermediates [33, 34]. Thus, to determine whether pTE15 generates ssDNA intermediates during plasmid replication, we conducted a Southern blot analysis of pTC82-RO and pTE15 plasmids. Recombinant pTC82-RO was selected as a positive ssDNA control based on our previous study [19]. As expected, ssDNA was detected in the culture of pTC82-RO grown with rifampicin and was degraded when S1 nuclease was added to the cell lysates (Fig. 6A). In contrast, for pTE15, no single-stranded pTE15 DNA was detected, even after prolonged exposure to the film (Fig. 6B). Thus, the absence of single-stranded intermediate DNA of pTE15 indicated that this plasmid did not replicate via the RCR mechanism.
Detection of ssDNA from pTC82-RO (A) and pTE15 (B) by Southern hybrisization. Total DNA from pTC82-RO or pTE15, grown with (+) or without (−) rifampicin, was electrophoresed on a 0.8% agarose gel with (+) or without (−) prior S1 nuclease treatment. Arrow indicated the position of ssDNA intermediates. Size standards of supercoiled DNA are indicated on the left
Dissection for class B form of theta replication
The data in Figs. 3 and 4 imply that pTE15 may employ a class B theta form of replication mechanism. The class B form of theta replication is known to be dependent on DNA polymerase I, and plasmid DNA yields can be elevated by adding chloramphenicol (Cm) to the culture medium to amplify plasmid copy numbers (PCN) [35, 36]. Therefore, we further determined variations in the PCN of pTE15 amplification with the addition of Cm to the culture (Table 2 and Supplementary Fig. 2). pUC19 as the positive control was transformed into E. coli TG1 and pTE15 was transformed into L. reuteri DSM 20016, when they grow in the mid-log phase, and then cultured with Cm (150 μg/mL). The plasmid amplification statuses of pUC19 and pTE15 were absolute quantification by qPCR. As expected, the PCN of pUC19 was considerably mild increased after the addition of Cm to the culture (Table 2). In contrast, the amplification efficiency of pTE15 was decreased by the addition of Cm to the culture, the PCN of pTE15 from 5.96 decreased to 2.35 after Cm treatment (Table 2).
Collectively, we demonstrated that the minireplicon of pTE15 contains the characteristics of IR, SDR, AT-rich (LDR, interon), DnaA box, and ColE1 cer-like sequences; however, this plasmid cannot be further amplified by adding Cm.
Stability of plasmids containing the cloned RR
To evaluate the stability of the cloned RR and original plasmid pTE15 (~ 15-kb), pTE15-RR (6.8-kb), and pTE15-RO (3.1-kb) were subjected to stability analysis (Fig. 7). After 216 generations of growth without antimicrobial selective pressure, 100% of Em-resistant colonies were observed for recombinant L. reuteri DSM 20016 containing pTE15. However, approximately 30% of these colonies were detected in recombinant L. reuteri containing pTE15-RR. In contrast, only 5% were observed for recombinant L. reuteri DSM 20016 containing pTE15-RO after growing for approximately 144 generations (Fig. 7).
Discussion
Most small and multi-copy plasmids of gram-positive bacteria are known to replicate via an RCR mechanism, and these RCR plasmids replicate with ssDNA intermediates. In contrast, most gram-negative bacteria replicate via the theta mechanism. In the present study, pTE15 was found to be a ~ 15-kb large plasmid from L. reuteri N16, suggesting that it may replicate via the theta mode. This study describes the characterization of pTE15 and showed that this large, naturally occurring plasmid (~ 15-kb) replicates via another theta mode mechanism. To our knowledge, although the characteristics of six theta-type plasmids belonged to lactobacilli were description thus for [20,21,22,23,24], but they almost could be classified as class A replicon, which must the plasmid encoded the Rep protein with a related origin of replication and replicate independently of DNA polymerase I [25]. Therefore, this is the first study demonstrating an unclassified class replicon of theta plasmid in a Lactobacillus species. The evidence for theta replication of pTE15 was based on the following observations: (1) unlike RCR plasmids, pTE15 did not generate any ssDNA replication intermediates; (2) the size of this plasmid was > 10 kb; (3) the ori consists of 8 IR, 3 SDR, an AT-rich (3 LDR) and 6 DnaA sequence; (4) it did not contain any features consistent with RCR. For example, it did not contain the DSO and rep genes and showed no SSO structure; (5) the essential replication motif of pTE15 does not contain a typical replication protein, Rep, and (6) its PCN was decreased by Cm treatment.
Six classes of theta-type plasmids can be distinguished based on their mode of replication initiation, including class A to F [15,16,17, 26]. Class A theta plasmids include R1, RK2, R6K, pSC101, pPS10, F, and P. These plasmids depend on plasmid encoded Rep proteins for replication initiation [37]. Class B theta plasmids include pColE1 and pColE1-like plasmids, and these plasmids can be amplified by adding Cm to the medium during the mid-log growth phase of bacteria. Most importantly, these plasmids rely exclusively on host factors for both double-strand melting and primer synthesis; thus, their replication does not depend on plasmid-encoding Rep proteins [38]. Class C plasmids include pColE2 and pColE3, and their replication depends on the Rep protein. Moreover, the Rep protein in class C plasmids displays primase activity, synthesizing a unique primer RNA (ppApGpA) that is extended by DNA polymerase I at a fixed site in the origin region [39]. Class D includes large, low-copy streptococcal plasmids that replicate in a broad range of gram-positive bacteria, including pAMβ1 from Enterococcus faecalis, pIP501 from Streptococcus agalactiae, and pSM19035 from Streptococcus pyogenes [40]. Replication of these plasmids also requires the plasmid-encoding Rep protein and DNA polymerase I [41]. Class E was represented by plasmid pLS20 from Bacillus subtilis that are not yet to be characterized [42]. Finally, class F must a replication initiation protein (RepN) but lack AT-rich region at replication initiation site and had multiple interons (DR) on the coding sequence of RepN. Its replication is independent of DNA polymerase I, such as pLJ1 of Lactobacillus sp., pAD1, pCF10, pPD1 and so on [25].
Based on the above classifications and characteristics of the six classes of theta replication, pTE15 could not be classified into either of them. Thus, we suggest that pTE15 be classified into a new theta mode of replication. The characteristics of this new theta replicon of pTE15 are discussed below.
According to our genetic analysis of pTE15 ori, the replicon of pTE15 contained unique features. For example, the iterons region with AT-rich sequences should be the ori; however, there were quite a few long sequences of IR around these AT-rich regions (Fig. 3A). These features have not been reported in current publications. For example, most of the motifs in the AT-rich region are DR of most prokaryotic plasmids, although there are exceptions such as RK2 the oriV origin, or Thermotoga maritima, where one of the motifs is inverted in relation to the others [43]. Another feature of pTE15 is that the essential ori is only about 1.3-kb (pTE15-RRΔ7, Table 1 and Fig. 4), which is not long enough to be translated for 60 amino acid residues (Fig. 3B). To support this, genetic analyses demonstrated that there was no ORF in this region. Moreover, pTE15 did not only generate any ssDNA replication intermediates but also this PCN could be decreased by adding Cm to the culture medium after mid-log phase. Thus, pTE15 cannot be classified under the class B mode of theta replication. However, according to our genetic analysis of pTE15 ori, it contains 6 DnaA box and an AT-rich (iterons, LDR) sequence that resembles an oriA-like structure. Collectively, the unique features of the minireplicon of pTE15 imply that this plasmid is a narrow-host-range plasmid. To support this, we showed that pTE15 was found in recombinant L. reuteri DSM 20016 at a copy number of approximately 6.0 per chromosome equivalent by qPCR. Furthermore, pTE15-RO functions only in L. reuteri DSM 20016 and L. fermentum PO2 bacterial strains, but does not function in several bacterial strains, including L. acidophilus ATCC 43258, L. plantarum ATCC 14917, L. lactis ATCC19435, B. subtilis RM125, B. circulans ATCC 21738, S. epidermidis BO95, S. aureus CNCTC 99/85, and E. faecalis JH2–2 [18]. Thus, pTE15 features as a low-copy number and narrow-host-range plasmid.
Altogether, our present data strongly suggest that pTE15 can be classified into the theta mode of replication, but it does not match the six previously known classes of theta mode replication mechanisms. Interestingly, the class E pLS20 plasmid from the gram-positive bacterium B. subtilis is a prototype conjugative plasmid, and its replication is shown to be fundamentally different from that of other plasmids in that it is unusually small (∼1.1-kb) and does not contain a typical replication gene [44]. Moreover, pLS20-derived replicons were established in a Pol I-mutated strain, and its copy number was not affected by Cm treatment. Therefore, the replication of pTE15 here is similar to that of pLS20, but pTE15 also contains unique features that have not been observed in the present study, as explained elsewhere. However, there is currently no DNA polymerase I mutant strain for L. reuteri, and thus, whether the host DNA polymerase I is involved in it replication initiation remains unclear.
Genetic analysis of pTE15 showed that the upstream of ori encoded 112 amino acid sequence of truncated RepA protein (TRepA) shared 99% identity with C-terminal residuals of Rep proteins of pRK11281 (Accession No. WP_016497314, unpublished) in Limosilactobacillus reuteri. The downstream of ori contained RepB (295 aa) and RepC (123 aa) were also had 99% identities with AAA family ATPase (Accession No. WP_098035017) and replication-associated protein RepC (WP_098035018), respectively in Limosilactobacillus antri (unpublished). Although TRepA, RepB and RepC were associated with pTE15 replication but these putative proteins were not essential for pTE15 replication. Otherwise when compared RepB and RepC of pTE15 with RepB and RepC of pAD1 using Blastp [29], which previous had been functional study [29], the similarity for amino acids was approximately 57 and 32%, respectively. The RepB involved in plasmid copy control and RepC may be involved in stable inheritance [29]. Thus, the functions of RepB and RepC in pTE15 could be similar to those of RepB and RepC in pAD1. Our stability analysis indicated that pTE15 is quite stable because it grew for 216 generations with 100% stability, without antimicrobial selective pressure. In contrast, only 5% of Em-resistant colonies were observed for L. reuteri DSM 20016 containing pTE15-RO after growing for approximately 144 generations. Since pTE15-RO lacks the complete repC sequence, these results imply that the repC of pTE15 is also involved in the stability of pTE15.
Conclusions
In conclusion, we described the features of an indigenous pTE15 plasmid from L. reuteri N16, and our data suggest that this plasmid should be classified as a distinct theta-type class in lactobacilli.
Methods
Bacteria and growth conditions
All the bacterial strains, indigenous plasmids, and recombinant plasmids used in the study are listed in Table 1. Briefly, Lactobacillus reuteri DSM 20016, a type of strain that contains no plasmid and also possesses Cm sensitive (Cms), Em sensitive (Ems), and tetracycline sensitive (Tets) abilities was selected and grown according to a previous study [3]. Escherichia coli TG1 (New England Biolabs) was cultured in LB broth (Luria-Bertani; Gibco) at 37 °C. When required, ampicillin (100 μg/mL; Sigma) and Em (10 μg/mL for Lactobacillus and 150 μg/mL for E. coli; Sigma) were added to the culture media. Notably, L. reuteri DSM 20016 transformants were selected for growth on MRS (De Man, Rogosa and Sharpe; Gibco) with Em (10 μg/mL) to contain a cloned fragment on which the replication function was located. Stocks of bacterial strains were maintained in 15% glycerol at − 80 °C. Other information regarding the recombinant plasmids is described in detail in Table 1.
Plasmid extraction and electrophoresis
Molecular manipulations, such as genome and plasmid isolation, electrophoresis, restriction endonuclease digestion, and fragment ligation, were performed according to standard techniques [27]. Large-scale plasmid DNA was purified using two successive cesium chloride (CsCl) density gradients [27]. Gel electrophoresis was performed on 0.8% agarose gel in TAE buffer (40 mM Tris-acetate, 1 mM EDTA; pH 7.0) at 5 V/cm.
Plasmid constructions and confirmation of the existence of minireplicon
The characteristics of the plasmids used, including pUC19, pUE80 (−), and pTE15, pTE15-RO, were generated as described in our previous study (Lin and Chung 1999). Briefly, to generate pTE15/19, pTE15 (~ 15.0 kb) was digested with XbaI, and pUC19 was digested with XbaI and calf-intestinal alkaline phosphatase. The two plasmids were cloned to obtain pTE15/19 (Fig. 1). Next, according to the mapping of pTE15 (Fig. 1), pTE15/19 was further digested with BsrBI, and the obtained 6.8-kb fragment was subcloned into pUE80 (−) that had been digested with HincII. The resulting recombinant plasmid was named pTE15-RR. pTE15-RR was then electroporated into L. reuteri DSM 20016 and screened using Em (10 μg/mL). Because the recombinant L. reuteri DSM 20016 with pTE15-RR could survive treatment with Em, it was digested with pTE15-RR and XbaI/PstI to generate a 3.1-kb fragment. This fragment was further subcloned to generate pTE15-RO, which contains the potential replication region (RR), and was subcloned into five fragments (A–E) for DNA sequencing (Fig. 1) [18]. To further confirm which fragments may contain the needed regions of replication origins, pTE15-RO was digested and subcloned as described below.
To examine and characterize its minireplicon, a deletion approach was employed in the present study. Briefly, pTE15-RO was further digested using different restriction enzymes to delete one of its seven fragments, generating pTE15-RRΔ1 to pTE15-RRΔ7 (Fig. 4). For example, the pTE15-RRΔ1to RRΔ7 regions were deleted using BsrBI-BglII, BsrBI-EcoRV, BsrBI-BamHI, HindIII-PstI, SacI-PstI, SacI-PvuI, and HindIII-BamHI, respectively (Table 1). Then, pTE15-RRΔ1 with different deleted regions was further subcloned into pUE80 (−) and then transformed into E. coli TG1 to confirm the correctness of these fragments. Then, these recombinant plasmids were purified and electroporated into L. reuteri DSM 20016 and screened using Em (10 μg/mL) to confirm which pTE15-RRΔ1 - RRΔ7 was responsible for replication. For instance, if recombinant L. reuteri DSM 20016 with transformed pTE15-RRΔ1 can grow under Em treatment, then pTE15-RRΔ1 contains the machinery of the RR. The integrity of the modified fragments in each constructed plasmid derivative was confirmed using DNA sequence analysis.
Analysis of ssDNA intermediate production
Single strand DNA was detected in the total DNA of L. reuteri transformed with pTE15 according to our previous study [19].
DNA sequencing
Universal primer was used according to the procedures described in the Sequenase 2.0 enzyme kit (US Biochem). Analysis of DNA sequence with gene finding and pattern recognition was performed using Blastx and GCG Wisconsin Package (UWGCG) provided by the Genetics Computer Group [29, 31].
Determination of absolute plasmid copy number
The plasmid copies number (PCN) of pUC19 and pTE15 were measured using quantitative real-time PCR (qPCR). Plasmid pUC19 was extracted from E. coli TG1 using the QIAGEN Plasmid Mini kit (Qiagen) and total DNA was extracted from E. coli contained pUC19 and L. reuteri DSM 20016 contained pTE15 using the QIAamp DNA Mini kit (Qiagen) and the method reported by Alimolaei and Golchin [45], respectively. The high purity of pUC19 was quantified by NanoPhotometer N60 (IMPLEN), then diluted continuously and the 10-fold dilution (101–107 copies/μL) was utilized to construct standard curves of bla for absolute quantification (Supplementary Fig. 2). Amplification and detection single copy genes were bla, dxs, repB, and alr of pUC19, E. coli, pTE15, and L. reuteri, respectively. The PCN of pUC19 and pTE15 were then calculated by dividing the copy number of bla and repB by the copy number of dxs and alr, respectively. All qPCRs were conducted three times, and the average revalues were used to calculate PCN.
Measurement of plasmid stability
A stability study was conducted according to a previous study with some modifications (Weaver, Clewell et al. 1993). Briefly, recombinant L. reuteri DSM 200016 containing pTE15, pTE15-RR, or pTE15-RO was grown overnight in MRS medium containing Em (10 μg/mL), which was further diluted 102-fold in antimicrobial-free MRS broth (time zero) and maintained in log phase from that point by periodic dilution every 10 generations (ca. 7.5 h). At each 36-generation interval (ca. 27 h), portions of the bacterial culture were analyzed for the percentage (%) of colonies that still maintained the plasmid-encoded Em resistance. All plasmid stability tests were conducted three times.
Availability of data and materials
the data (nucleotide sequence) is deposited on NCBI GenBank (Accession No. OP744582 and AF036766).
Abbreviations
- Em:
-
Erythromycin
- Cm:
-
Chloramphenicol
- Tet:
-
Tetracyclin
- DSO:
-
Double-strand origin
- SSO:
-
Single-strand origin
- RCR:
-
Rolling-circle replication
- RBS:
-
Ribosome binding sites
- SDR:
-
Short directed repeat
- LDR:
-
Long directed repeat
- IR:
-
Inverted repeat
- RR:
-
Replication region
- LAB:
-
Lactic acid bacteria
- PCN:
-
Plasmid copy number
References
Islam SU. Clinical uses of probiotics. Medicine. 2016;95(5):e2658.
Kechagia M, et al. Health benefits of probiotics: a review. ISRN. 2013;2013:481651.
Kandler O, Stetter K-O, Köhl R. Lactobacillus reuteri sp. nov., a new species of heterofermentative lactobacilli. Zentralblatt Für Bakteriologie: I Abt Originale C: Allgemeine, Angewandte Und Ökologische Mikrobiologie. 1980;1(3):264–9.
Chung T, et al. In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Ecol Health Dis. 1989;2(2):137–44.
Mu Q, Tavella VJ, Luo XM. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. 2018;9:757.
Liu Y, et al. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(5):G1087–96.
Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173(10):1327–37.
Bhogoju S, et al. Effects of Lactobacillus reuteri and Streptomyces coelicolor on growth performance of broiler chickens. Microorganisms. 2021;9(6):1341.
Heng N, Jenkinson HF, Tannock GW. Cloning and expression of an endo-1, 3-1, 4-beta-glucanase gene from Bacillus macerans in Lactobacillus reuteri. Appl Environ Microbiol. 1997;63(8):3336–40.
Seegers JF. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 2002;20(12):508–15.
Wu CM, Chung TC. Mice protected by oral immunization with Lactobacillus reuteri secreting fusion protein of Escherichia coli enterotoxin subunit protein. FEMS Immunol Med Microbiol. 2007;50(3):354–65.
Pouwels PH, Leer RJ. Genetics of lactobacilli: plasmids and gene expression. Antonie Van Leeuwenhoek. 1993;64(2):85–107.
von Wright A, Saarela M. A variant of the staphylococcal chloramphenicol resistance plasmid pC194 with enhanced ability to transform Lactococcus lactis subsp. lactis. Plasmid. 1994;31(1):106–10.
Fernández-Tresguerres M, et al. Host growth temperature and a conservative amino acid substitution in the replication protein of pPS10 influence plasmid host range. J Bacteriol. 1995;177(15):4377–84.
Bruand C, et al. A fourth class of theta-replicating plasmids: the pAM beta 1 family from gram-positive bacteria. Proc Natl Acad Sci. 1993;90(24):11668–72.
Lilly J, Camps M. Mechanisms of theta plasmid replication. Microbiology spectrum. 2015;3(1):3.1. 02.
Kim JW, et al. Mechanisms of Theta plasmid replication in Enterobacteria and implications for adaptation to its host. EcoSal Plus. 2020;9(1). PMID: 33210586.
Lin C-F, Chung T-C. Cloning of erythromycin-resistance determinants and replication origins from indigenous plasmids of Lactobacillus reuteri for potential use in construction of cloning vectors. Plasmid. 1999;42(1):31–41.
Lin C-F, Ho J-L, Chung T-C. Characterization of the replication region of the Lactobacillus reuteri plasmid pTC82 potentially used in the construction of cloning vector. Biosci Biotechnol Biochem. 2001;65(7):1495–503.
Terán LC, et al. Nucleotide sequence and analysis of pRC12 and pRC18, two theta-replicating plasmids harbored by Lactobacillus curvatus CRL 705. PLoS One. 2020;15(4):e0230857.
Alpert C-A, et al. Characterization of a theta-type plasmid from Lactobacillus sakei: a potential basis for low-copy-number vectors in lactobacilli. Appl Environ Microbiol. 2003;69(9):5574–84.
Asteri I-A, et al. Comparative and evolutionary analysis of plasmid pREN isolated from Lactobacillus rennini, a novel member of the theta-replicating pUCL287 family. FEMS Microbiol Lett. 2011;318(1):18–26.
Takiguchi R, et al. Complete nucleotide sequence and characterization of a cryptic plasmid from Lactobacillus helveticus subsp. jugurti. Appl Environ Microbiol. 1989;55(6):1653–5.
Kazi TA, et al. Plasmid-based gene expression Systems for Lactic Acid Bacteria: a review. Microorganisms. 2022;10(6):1132.
Émond É, et al. Molecular characterization of a theta replication plasmid and its use for development of a two-component food-grade cloning system for Lactococcus lactis. Appl Environ Microbiol. 2001;67(4):1700–9.
Johnson M, et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 2008;36(suppl_2):W5–9.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual: Cold spring harbor laboratory press; 1989.
Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–19.
Weaver KE, Clewell D, An F. Identification, characterization, and nucleotide sequence of a region of Enterococcus faecalis pheromone-responsive plasmid pAD1 capable of autonomous replication. J Bacteriol. 1993;175(7):1900–9.
Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12(1Part1):387–95.
Luo H, Gao F. DoriC 10.0: an updated database of replication origins in prokaryotic genomes including chromosomes and plasmids. Nucleic Acids Res. 2019;47(D1):D74–7.
Te Riele H, Michel B, Ehrlich S. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986;5(3):631–7.
Gruss A, Ehrlich SD. The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Rev. 1989;53(2):231–41.
Clewell DB. Nature of col E1 plasmid replication in Escherichia coli in the presence of chloramphenicol. J Bacteriol. 1972;110(2):667–76.
Morlon J, Cavard D, Lazdunski C. Physical map of pColA-CA31, an amplifiable plasmid, and location of colicin a structural gene. Gene. 1982;17(3):317–21.
Yao F, et al. Detection and characterization of a theta-replicating plasmid pLP60 from Lactobacillus plantarum PC518 by inverse PCR. Heliyon. 2019;5(8):e02164.
Mukhopadhyay G, Chattoraj DK. Conformation of the origin of P1 plasmid replication: initiator protein induced wrapping and intrinsic unstacking. J Mol Biol. 1993;231(1):19–28.
Wu Y-C, Liu S-T. A sequence that affects the copy number and stability of pSW200 and ColE1. J Bacteriol. 2010;192(14):3654–60.
Takechi S, Matsui H, Itoh T. Primer RNA synthesis by plasmid-specified rep protein for initiation of ColE2 DNA replication. EMBO J. 1995;14(20):5141–7.
Bruand C, Ehrlich SD. Transcription-driven DNA replication of plasmid pAMβ1 in Bacillus subtilis. Mol Microbiol. 1998;30(1):135–45.
Le Chatelier E, et al. The RepE initiator is a double-stranded and single-stranded DNA-binding protein that forms an atypical open complex at the onset of replication of plasmid pAMβ1 from gram-positive bacteria. J Biol Chem. 2001;276(13):10234–46.
Meijer WJ, et al. Characterization of the replication region of the Bacillus subtilis plasmid pLS20: a novel type of replicon. Nucleic Acids Res. 1995;23(16):3214–23.
Rajewska M, Wegrzyn K, Konieczny I. AT-rich region and repeated sequences–the essential elements of replication origins of bacterial replicons. FEMS Microbiol Rev. 2012;36(2):408–34.
Val-Calvo J, et al. pLS20 is the archetype of a new family of conjugative plasmids harboured by Bacillus species. NAR Genom Bioinform. 2021;3(4):lqab096.
Alimolaei M, Golchin M. An efficient DNA extraction method for Lactobacillus casei, a difficult-to-lyse bacterium. Int J Enteric Pathog. 2016;4(1):e32472.
Acknowledgments
The authors wish to acknowledge the help of Shin-Ji Hsu from the Instutute of Veterinary Medicine, National Pingtung University of Science and Technology for plasmid copy number measurement. In addition, the authors would like to thank Editage (ISO/IEC: 27001:2013 certified for quality) for providing English editing services. We would like to thank anonymous (unknown) reviewers and the editor for their comments.
Funding
This research received no external founding.
Author information
Authors and Affiliations
Contributions
P. W. Chen conceived the idea and performed experiments. P. W. Chen and C. F. Lin wrote, reviewed, edited, and revised the manuscript. The author(s) read approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NA.
Consent for publication
NA.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Alignment of pTE15 replication region (RR) with non-redundant sequences in NCBI was blast tree by Tree Viewer 1.19.3. The blast tree shown the most similarity plasmid is pLRI04 and the nucleotides (1–1534-bp) at the upstream of iteron (LDR, AT-rich) of pTE15 has 95.8% identities with plasmid pLRI04 of L. reuteri I5007 isolated from health pig (unpublished data). Supplementary Figure 2. Construction of the standard curve for bla and confirmation of qPCR amplification specificities of bla, dxs, repB, and alr. (A) The standard curves were constructed with seial 10-fold dilutions of the pUC19, ranged from 1 × 101 to 1 × 107 copies/uL. Each standard dilution was amplified by real-time qPCR using bla-set in eight duplication. For each gene, determined CT values were plotted against the logarithm of their known initial copy numbers (n = 3). A standard curve was generated by linear regression through these points. (B - E) Melting peaks were examined for the bla-, dxs-, repB-, and the alr-sets with a quantitative standard sample, E. coli and L. reuteri total DNA samples before and after Cm treatments as templates. The melting temperatures were in the panels B to E. Each DNA sample was amplified by real-time qPCR in triplicate.
Additional file 2: Figure S6.
(A) of original blots were from the red box with dash-line in lines 3–4 and lines 7–8 of panel C. (B) of original blots were from the blue box with dash-line in lines 1–2 and lines 3–4 of panel D. In panel D, lines 5–8 were from RCR-type plasmid pC194 of Staphylococcus aureus, which was not included in this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, PW., Lin, CF. Characterization of a novel theta-type replicon of indigenous plasmid pTE15 from Lactobacillus reuteri N16. BMC Microbiol 22, 298 (2022). https://doi.org/10.1186/s12866-022-02718-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02718-4