Abstract
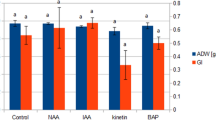
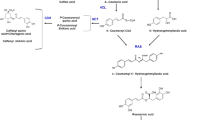
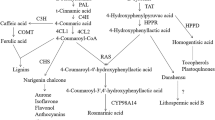
The induction of several secondary metabolites in plants is one of the most commonly observed effects after the external addition of methyl jasmonate (MeJA). After the elicitation of Catharanthus roseus hairy roots with different concentrations of MeJA, changes in the accumulation of alkaloids such as ajmalicine, serpentine, ajmaline and catharanthine were observed. In addition to the increased accumulation of alkaloids in the tissues, the root exudation of phytochemicals increased compared to that of the non-treated control hairy roots. Moreover, MeJA induced differential secretion of several C. roseus hairy root metabolites.





Similar content being viewed by others
References
Vick, B. A., & Zimmerman, D. C. (1984). The biosynthesis of jasmonic acid by several plant species. Plant Physiology, 75, 458–461.
Feussner, I., & Wasternack, C. (2002). The lipoxygenase pathway. Annual Review of Plant Biology, 53, 275–297. doi:10.1146/annurev.arplant.53.100301.135248.
Wasternack, C. (2007). Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany, 100, 681–697. doi:10.1093/aob/mcm079.
Creelman, R. A., & Mullet, J. E. (1997). Biosynthesis and action of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 355–381. doi:10.1146/annurev.arplant.48.1.355.
Farmer, E. E., Almeras, E., & Krishnamurthy, V. (2003). Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Current Opinion in Plant Biology, 6, 372–378. doi:10.1016/S1369-5266(03)00045-1.
Creelman, R. A., & Mullet, J. E. (1995). Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proceedings of the National Academy of Sciences of the United States of America, 92, 4114–4119. doi:10.1073/pnas.92.10.4114.
Badri, D. V., Loyola-Vargas, V. M., Du, J., Stermitz, F. R., Broeckling, C. D., Iglesias-Andreu, L. G., et al. (2008). Increase in the secretion of phytochemicals by roots of Arabidopsis thaliana under the effect of several growth regulators. The New Phytologist, 179, 209–223. doi:10.1111/j.1469-8137.2008.02458.x.
Vázquez-Flota, F., Moreno-Valenzuela, O. A., Miranda-Ham, M. L., Coello-Coello, J., & Loyola-Vargas, V. M. (1994). Catharanthine and ajmalicine synthesis in Catharanthus roseus hairy root cultures. Medium optimization and elicitation. Plant Cell, Tissue and Organ Culture, 38, 273–279. doi:10.1007/BF00033887.
Aerts, R. J., Gisi, D., De Carolis, E., De Luca, V., & Baumann, T. W. (1994). Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloid in Catharanthus and Cinchona seedling. The Plant Journal, 5, 635–643. doi:10.1111/j.1365-313X.1994.00635.x.
Gantet, P., Imbault, N., Thiersault, M., & Doireau, P. (1998). Necessity of a functional octadecanoic pathway for indole alkaloid synthesis by Catharanthus roseus cell suspensions cultured in an auxin-starved medium. Plant & Cell Physiology, 39, 220–225.
Lee-Parsons, C. W. T., & Ertük, S. (2005). Ajmalicine production in methyl jasmonate-induced Catharanthus roseus cell cultures depends on Ca2+ level. Plant Cell Reports, 24, 677–682. doi:10.1007/s00299-005-0026-0.
Lee-Parsons, C. W. T., Ertük, S., & Tengtrakool, J. (2004). Enhancement of ajmalicine production in Catharanthus roseus cell cultures with methyl jasmonate is dependent on timing and dosage of elicitation. Biotechnology Letters, 26, 1595–1599. doi:10.1023/B:BILE.0000045825.37395.94.
Van der Fits, L., Zhang, H., Menke, F. L. H., Deneka, M., & Memelink, J. (2000). A Catharanthus roseus BPF-1 homologue interacts with an elicitor-responsive region of the secondary metabolite biosynthetic gene Str and is induced by elicitor via a JA-independent signal transduction pathway. Plant Molecular Biology, 44, 675–685. doi:10.1023/A:1026526522555.
Menke, F. L. H., Champion, A., Kijne, J. W., & Memelink, J. (1999). A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. The EMBO Journal, 18, 4455–4463. doi:10.1093/emboj/18.16.4455.
Van der Fits, L., & Memelink, J. (2000). ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science, 289, 295–297. doi:10.1126/science.289.5477.295.
Zhang, Z.-P., & Baldwin, I. T. (1997). Transport of [2–14C] jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta, 203, 436–441. doi:10.1007/s004250050211.
Parchmann, S., Gundlach, H., & Mueller, M. J. (1997). Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiology, 115, 1057–1064. doi:10.1104/pp.115.3.1057.
De Luca, V., & St Pierre, B. (2000). The cell and developmental biology of alkaloid biosynthesis. Trends in Plant Science, 5, 168–173. doi:10.1016/S1360-1385(00)01575-2.
Loyola-Vargas, V. M., Broeckling, C. D., Badri, D. V., & Vivanco, J. M. (2007). Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta, 225, 301–310. doi:10.1007/s00425-006-0349-2.
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., & Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with pand other organisms. Annual Review of Plant Biology, 57, 233–266. doi:10.1146/annurev.arplant.57.032905.105159.
Ciau-Uitz, R., Miranda-Ham, M. L., Coello-Coello, J., Chí, B., Pacheco, L. M., & Loyola-Vargas, V. M. (1994). Indole alkaloid production by transformed and non-transformed root cultures of Catharanthus roseus. In Vitro Cell Developmental Biology-Plant, 30, 84–88.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50, 151–158. doi:10.1016/0014-4827(68)90403-5.
Monforte-González, M., Ayora-Talavera, T., Maldonado-Mendoza, I. E., & Loyola-Vargas, V. M. (1992). Quantitative analysis of serpentine and ajmalicine in plant tissues of Catharanthus roseus and hyoscyamine and scopolamine in root tissues of Datura stramonium by densitometry in thin layer chromatography. Phytochemical Analysis, 3, 117–121. doi:10.1002/pca.2800030305.
Matsumoto, T., Ikeda, T., Okimura, C., Obi, Y., Kisaki, T., Noguchi, M. (1982). Production of ubiquinone 10 (UQ-10) by UQ highly producing strains selected by a cell cloning technique. In A. Fujiwara (Ed.), Plant tissue culture (pp. 275–276). Tokyo: Japanese Association for Plant Tissue Culture.
Loyola-Vargas, V. M., Galaz-Avalos, R. M., & Rodríguez-Ku, J. R. (2007). Catharanthus biosynthetic enzymes: The road ahead. Phytochemistry Reviews, 6, 307–339. doi:10.1007/s11101-007-9064-2.
Spollansky, T. C., Pitta-Alvarez, S. I., & Giulietti, A. M. (2000). Effect of jasmonic acid and aluminum on production of tropane alkaloids in hairy root cultures of Brugmansia candida. Electronic Journal of Biotechnology, 3, 72–75.
Colque, R., Viladomat, F., Bastida, J., & Codina, C. (2004). Improved production of galanthamine and related alkaloids by methyl jasmonate in Narcissus confusus shoot-clumps. Planta Medica, 70, 1180–1188. doi:10.1055/s-2004-835849.
Zhao, J., Davis, L. C., & Verpoorte, R. (2005). Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology Advances, 23, 283–333. doi:10.1016/j.biotechadv.2005.01.003.
Bonfill, M., Bentebibel, S., Moyano, E., Palazón, J., Cusidó, R. M., Eibl, R., et al. (2007). Paclitaxel and baccatin III production induced by methyl jasmonate in free and immobilized cells of Taxus baccata. Biologia Plantarum, 51, 647–652. doi:10.1007/s10535-007-0137-2.
Gadzovska, S., Maury, S., Delaunay, A., Spasenoski, M., Joseph, C., & Hagège, D. (2007). Jasmonic acid elicitation of Hypericum perforatum L. cell suspensions and effects on the production of phenylpropanoids and naphtodianthrones. Plant Cell, Tissue and Organ Culture, 89, 1–13. doi:10.1007/s11240-007-9203-x.
Baldwin, I. T. (1996). Methyl jasmonate-induced nicotine production in Nicotiana attenuata: inducing defenses in the field without wounding. Entomologia Experimentalis et Applicata, 80, 213–220. doi:10.1007/BF00194760.
Staswick, P. E., Su, W., & Howell, S. H. (1992). Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proceedings of the National Academy of Sciences of the United States of America, 89, 6837–6840. doi:10.1073/pnas.89.15.6837.
Goossens, A., Hakkinen, S. T., Laakso, I., Seppanen-Laakso, T., Biondi, S., De Sutter, V., et al. (2003). A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 8595–8600. doi:10.1073/pnas.1032967100.
El-Sayed, M., & Verpoorte, R. (2005). Methyljasmonate accelerates catabolism of monoterpenoid indole alkaloids in Catharanthus roseus during leaf processing. Fitoterapia, 76, 83–90. doi:10.1016/j.fitote.2004.10.019.
Eichhorn, H., Klinghammer, M., Becht, P., & Tenhaken, R. (2006). Isolation of a novel ABC-transporter gene from soybean induced by salicylic acid. Journal of Experimental Botany, 57, 2193–2201. doi:10.1093/jxb/erj179.
Acknowledgements
We are grateful to Emily Wortman-Wunder and Clelia De-la-Peña for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-May, E., Galaz-Ávalos, R.M. & Loyola-Vargas, V.M. Differential Secretion and Accumulation of Terpene Indole Alkaloids in Hairy Roots of Catharanthus roseus Treated with Methyl Jasmonate. Mol Biotechnol 41, 278–285 (2009). https://doi.org/10.1007/s12033-008-9111-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9111-2