Abstract
CircRNAs, a type of non-coding RNA widely present in eukaryotic cells, have emerged as a prominent focus in tumor research. However, the functions of most circRNAs remain largely unexplored. Known circRNAs exert their regulatory roles through various mechanisms, including acting as microRNA sponges, binding to RNA-binding proteins, and functioning as transcription factors to modulate protein translation and coding. Tumor growth is not solely driven by gene mutations but also influenced by diverse constituent cells and growth factors within the tumor microenvironment (TME). As crucial regulators within the TME, circRNAs are involved in governing tumor growth and metastasis. This review highlights the role of circRNAs in regulating angiogenesis, matrix remodeling, and immunosuppression within the TME. Additionally, we discuss current research on hypoxia-induced circRNAs production and commensal microorganisms’ impact on the TME to elucidate how circRNAs influence tumor growth while emphasizing the significance of modulating the TME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Cancer is often referred to as “a wound that never heals”, characterized by the uncontrolled proliferation of malignant cells alongside impaired immune system function. Abnormal expression or mutation of oncogenes/cancer suppressor genes leads to heterogeneity among normal cells, transforming them into malignancy [1]. In the early stages of tumor development, lymphocytes recognize surface antigens expressed by cancer cells and eliminate them through immune responses. To evade immune surveillance effectively, cancer cells shed surface antigens while secreting cytokines that recruit immune-suppressor cells for suppressing immune responses [2, 3].
The “seed and soil” hypothesis, proposed by Stephen Paget in 1889, suggests that the interaction between tumor cells (the seed) and their microenvironment (the soil) is crucial [4]. The TME is composed of various cellular components such as immune cells, tumor-associated endothelial cells (CAEs), tumor-associated fibroblasts (CAFs), pericytes, etc., as well as non-cellular components including cytokines, growth factors, metabolic substances, and extracellular matrix (ECM) proteins [5]. Rapid tumor growth triggers environmental changes, such as hypoxia and acidosis, which disrupt coordinated cellular interactions, leading to ECM remodeling, the induction of angiogenesis, and the inhibition of immune response. Consequently, a heterogeneous ecological environment conducive to cancer development is established [5, 6].
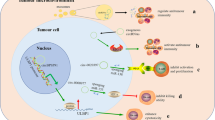
In recent years, circRNAs have become a research hotspot in cancer studies. There are complex regulatory interactions between circRNAs and TME components [7,8,9,10]. These findings provide ideas and theoretical basis for developing new cancer treatment methods. This review highlights the role of circRNAs in regulating angiogenesis, matrix remodeling, and immunosuppression within the TME. Additionally, we discuss current research on hypoxia-induced circRNAs production and commensal microorganisms’ impact on the TME to elucidate how circRNAs influence tumor growth while emphasizing the significance of modulating the TME (Fig. 1).
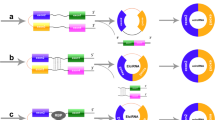
CircRNAs’ biogenesis and functions: A CircRNAs are formed by backsplicing linear RNA molecules at their 5′ and 3′ ends. B CircRNAs function as competitive endogenous RNA molecules, sequestering miRNAs. C CircRNAs regulate gene transcription and expression. D Interact with RNA-binding proteins. E CircRNAs can encode proteins driven by IRES
CircRNAs functions
Compared to traditional linear RNAs, circRNAs are characterized by their closed-loop structure, which confers resistance to RNA exonucleases and enables abundant and stable expression in cells and body fluids. With the development of RNA sequencing, thousands of circRNAs have been found in mammalian cells. Initially thought to be by-products of shearing, circRNAs play an important role in regulating the onset and development of many diseases [11, 12]. Current studies show that their functions can be divided into four parts: (1) Acting as miRNA sponges or repositories [13, 14]. (2) Interacting with RNA-binding proteins [15, 16]. (3) Functioning as translated proteins/peptides [17, 18]. And (4) regulating gene transcription and expression [19, 20]. A majority of dysregulated circRNAs have emerged as crucial regulators in cancer progression by modulating numerous cancer-associated molecules, thereby promoting tumorigenesis, suppressing tumor immunity, inducing angiogenesis, facilitating invasion and metastasis. Furthermore, circRNAs exhibit aberrant expression patterns in various diseases and govern disease progression encompassing cardiovascular diseases, autoimmune disorders, and inflammation [21,22,23] (Fig. 2).
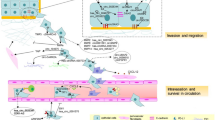
Roles of circRNAs in regulating the tumor microenvironment. A The chemotactic factors in the TME recruit lymphocytes and myeloid cells, which then differentiate into immunosuppressive cells under the influence of cytokines. B The exosomal circRNAs derived from immune-suppressive cells promote tumor growth and regulate angiogenesis. C CircRNAs derived from tumors induce polarization of macrophages and suppress the tumor-killing activity of TILs. D The exosomal circRNAs derived from CAFs promote tumor growth, regulate extracellular matrix remodeling, and modulate immune cell activity. E CircRNAs derived from tumor symbiotic microorganisms promote tumor growth and EMT. F Under hypoxic conditions, tumor-derived circRNAs regulate glucose metabolism through the glycolytic pathway, promote tumor growth and angiogenesis by modulating the expression of HIF-1a
CircRNAs regulate angiogenesis in the tumor microenvironment
Angiogenesis, a hallmark of cancer, facilitates the provision of adequate nutrition and removal of metabolic wastes from tumors. Stimulated by hypoxia and nutrient deficiency, tumor cells release vascular endothelial growth factor (VEGF) to promote germination and proliferation of endothelial cells [24]. Additionally, constituent cells of the TME, including TAMs, mesenchymal stem cells (MSCs), and CAFs, contribute to tumor angiogenesis by releasing substantial amounts of pro-angiogenic signals [25, 26].
The main mechanism by which circRNAs regulate angiogenesis is through the ceRNA network, with VEGF being one of its major targets. For instance, in colorectal cancer, circ_0056618 enhances angiogenesis by binding to miR-206 and subsequently upregulating CXCR4 and VEGFA expression [27]. In glioblastoma stem cells (GSCs), circARF1 upregulates ISL2 expression via sponge adsorption of miR-342-3p, which in turn regulates VEGFA expression. This promotes endothelial cell proliferation and angiogenesis through the VEGFA-mediated ERK signaling pathway [28]. Moreover, up-regulation of circSHKBP1 exosomes in gastric cancer enhances VEGF mRNA stability by regulating human antigen R (HUR) expression. Consequently, this promotes gastric cancer angiogenesis [29].
Furthermore, circRNAs also regulate angiogenesis through the modulation of other gene expressions. In colorectal cancer (CRC), circ3823 acts as a competitive endogenous RNA for miR-30c-5p to alleviate its inhibitory effect on TCF7. As a result, MYC and CCND1 are upregulated leading to CRC progression and angiogenesis [30].Circ-CCAC1 derived from extracellular vesicles disrupts endothelial barrier integrity when translocated onto endothelial monolayers thereby inducing angiogenesis in cholangiocarcinoma (CCA) [31]. In osteosarcoma, the YY1 transcription factor induces upregulation of circFIRRE and partially increases mRNA and protein levels of LUZP1 by adsorbing miR-486-3p and miR-1225-5p, promoting tumor cell growth and angiogenesis, thereby driving primary osteosarcoma progression and lung metastasis [32].
Notably, cancer cells can obtain blood supply through mechanisms independent of endothelial cell proliferation such as vascular co-option (VC) and vasculogenic mimicry (VM) [33]. These two processes provide an alternative blood supply for neovascularization in cancer. Therefore, they are considered the main reason for anti-angiogenic therapy failure in some cancers [34, 35]. CircRNAs may also have regulatory roles in VC and VM generation processes [36]. For instance, exosomal circRNA-100,338 affects the cell proliferation and VM-forming ability of human umbilical vein endothelial cells (HUVEC) to promote hepatocellular carcinoma metastasis both in vitro and in vivo experiments [37]. Additionally, circRNA-000284 was significantly upregulated in cervical cancer cells regulating SNAIL2 expression by directly targeting miR-506 the transcription factor SNAIL2 positively regulates L1CAM to promote cancer cell attachment to preexisting blood vessels spreading along them [38]. Moreover, cancer cells utilize the flexor foot, an actin-based cytoplasmic extension with high expression of CDC42 and CD44, to migrate along the outer surface of blood vessels. Silencing this mechanism inhibits vascular co-option (VC) by disrupting contact with pericytes. In renal clear cell carcinoma, androgen receptor promotes VC through modulation of circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signaling [39] (Table 1).
CircRNAs and extracellular matrix remodeling
The extracellular matrix (ECM), composed of various fibrous components (e.g., collagen, fibronectin, and elastin) and non-fibrous molecules (e.g., proteoglycans, hyaluronic acid, and glycoproteins), plays a crucial role as a tissue barrier against tumor invasion and metastasis. In the context of tumors, the precise regulation of ECM homeostasis is disrupted by cancer cells, cancer-associated fibroblasts (CAFs), immune cells, and other stromal cells. Perturbation and dysregulation of the ECM elicit diverse physiological signals that facilitate cancer cell proliferation and invasion [40, 41].
CircRNAs have been identified to modulate the expression of ECM components such as collagen proteins (e.g., COL5A1 and COL1A1) in multiple types of cancers. For instance, in breast cancer,circACAP2 targets specific miRNAs to promote breast tumor progression through regulating the expression of adsorbed miRNA-targeted genes like collagen proteins COL5A [42]. Similarly, in nasopharyngeal carcinoma, circ_0000523 exerts its function by sequestering miRNAs to regulate the expression levels of target genes including collagen proteins like COL1A [43]. In oral squamous cell carcinoma, hsa_circRNA_0001971 facilitates disease progression by adsorbing miR-186-5p to modulate the expression of fibronectin FNDC3B [44]. Additionally, the circ-FNDC3B derived from the FNDC3B gene plays a crucial role in various tumors such as gastric cancer, esophageal cancer, and colorectal cancer [45,46,47], it promotes tumor epithelial-mesenchymal transition (EMT), invasion, and metastasis by regulating E-cadherin and CD44. Furthermore, hsa_circ_0000285 and circ-CAMK2A enhance tumor growth and metastasis via upregulating FN1 expression through targeting specific adsorbed miRNAs in Gastric cancer and lung cancer respectively [48, 49]. Syndecan (SDC), an integral membrane protein involved in intercellular adhesion, signal transduction, and cell-matrix interactions via its extracellular matrix protein receptor, exhibits abnormal expression patterns across various cancers [50,51,52]. Circ_RPPH1 enhances SDC1 expression to drive glioma malignancy via sequestration of miR-627‐5p/miR‐663a [53], whereas circ_0058063 facilitates thyroid cancer progression by sponging miR‐330‐3p/SDC4 axis [54]. Additionally, hyaluronic acid(HA), which serves as a critical constituent within ECM regulating tissue stiffness, maintaining homeostasis, and functioning as a signaling molecule for multiple cellular subtypes, is known to promote tumorigenesis including proliferation, migration, invasion, and drug resistance via ECM remodeling within diverse neoplastic contexts [55,56,57]. In papillary thyroid cancer,circ_102002 acts as a miR-488-3p sponge, leading to the upregulation of hyaluronic acid synthetase 2 (HAS2), which in turn promotes EMT and cell migration [58].
Tumor cells and cancer-associated fibroblasts (CAFs) co-secrete various matrix-remodeling enzymes, including MMPs, ADAMs, and ADAMTs, which degrade extracellular matrix (ECM) components and release stromal factors and growth factors to promote cancer cell growth, metastasis, and angiogenesis [41].CircRNAs play crucial roles in regulating the expression of MMPs. For instance, circMMP1 promotes colorectal cancer growth and metastasis by sponging miR-1238 to upregulate the expression of MMP1/MMP2/MMP9 [59]. Hsa_circRNA_101996 enhances gastric cancer progression by upregulating MMP-2/MMP-9 via the miR-143/TET2 pathway [60]. High expression of ciRS-7 maintains the migratory and invasive properties of triple-negative breast cancer cells by acting as a competing endogenous RNA for miR-1299 to increase the expression of MMPs [61].
CircRNAs and CAFs
Cancer-associated fibroblasts (CAFs) play a pivotal role in the tumor microenvironment, engaging in extensive interactions with cancer cells and exerting influence on other TME components, including the extracellular matrix, angiogenesis, and immune infiltration. Fibroblasts are activated to transform into CAFs by various stimuli derived from tumors and immune-infiltrating cells, such as transforming growth factor β (TGF-β) family ligands, lysophosphatidic acid (LPA), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), interleukin-1 (IL-1), IL-6, and granulin [62, 63]. The abundance of CAFs is closely associated with the prognosis of different human tumors, while also governing therapeutic efficacy and serving as potential therapeutic targets themselves [64]. CircCUL2 is specifically expressed in CAFs. Upregulation of circCUL2 expression induces an activated CAF phenotype, which functions as a ceRNA and modulates the miR-203a-3p/MyD88/NF-κB/IL6 axis, then promotes the progression of pancreatic ductal adenocarcinoma (PDAC) by secreting IL-6 [65]. Cytokines derived from CAFs play a crucial role in tumor progression and metastasis by modulating the expression of circRNAs. In hepatocellular carcinoma, upregulated expression of circUBAP2 is observed in tumor tissues stimulated by CXCL11 secreted from CAFs. CircUBAP2 enhances the levels of IFIT1/3 and facilitates the expression of IL-17 and IL-1β through miR-4756 targeting, thereby enhancing the migratory capacity of hepatocellular carcinoma cells [66].
Additionally, CAFs play a crucial role in promoting tumor drug resistance, and their mechanism of resistance may be linked to the aberrant expression of circRNAs. Specifically, circZFR is highly expressed in CAFs and exosomes derived from CAFs. Elevated levels of circZFR have been shown to inhibit the STAT3/NF-κB pathway, thereby enhancing cisplatin resistance and promoting tumor growth [67]. In another study, it was found that CAFs can induce cancer stemness and gemcitabine resistance through leukemia inhibitory factor (LIF), which is promoted by circFARP1 specifically expressed in CAFs via direct binding to caveolin 1 (CAV1). Moreover, high levels of circFARP1 were positively associated with poorer survival and gemcitabine chemoresistance in pancreatic ductal adenocarcinoma patients due to its upregulation of LIF through sponge adsorption of miR-660-3p [68] (Table 2).
CircRNAs and hypoxia
Due to the rapid and uncontrolled proliferation of tumors, nearly all solid tumors exhibit typical microenvironmental features such as inadequate blood supply or hypoxia. Hypoxia, characterized by reduced oxygen supply, is a hallmark of tumor microenvironment, prompting tumor cells to reprogram their metabolism through cytokine regulation in response to the hypoxic environment. Meanwhile, hypoxia stimulates angiogenesis, regulates fibrinolytic activity, and suppresses immune cell function, thereby remodeling the TME [69,70,71].
Enhancement of the glycolytic pathway is an important manifestation of tumor metabolic reprogramming, as evidenced by the Warburg effect which indicates increased glycolysis even under aerobic conditions, providing sufficient energy and nutrients for tumor growth and proliferation [72]. Previous studies have shown that circRNAs mainly participate in regulating the glycolytic metabolism process under hypoxic conditions, with lactate dehydrogenase A (LDHA) being their main target. For example, circMAT2B enhances glycolysis to promote HCC progression through the circMAT2B/miR-338-3p/PKM2 axis [73]. CircARHGAP29 increases LDHA stability by enhancing the interaction between LDHA and IGFBP2, leading to enhanced glycolytic metabolism in prostate cancer. Additionally, circARHGAP29 interacts with and stabilizes c-Myc expression, further increasing LDHA by promoting its transcriptional expression [74]. Similarly, in PDAC, hypoxia-induced exosomal circPDK1 which regulates the miR-628-3p/BPTF axis and degrades BIN1 to promote c-Myc activation [75].
Increased expression of HIF-1α plays a pivotal role in cellular mechanisms triggered by hypoxia. In hypoxic conditions, activated HIF-1α regulates the activity of transcription factors to downregulate E-cadherin expression, thereby promoting epithelial-mesenchymal transition (EMT) [76]. Additionally, HIF-1α disrupts the expression of enzymes involved in collagen polymerization and alignment as well as integrin activity, facilitating tumor migration. Moreover, HIF-1α mediates vascular and lymphatic vessel leakage and compression, enabling metastatic cancer cells to traverse the vessel wall [77, 78]. CircRNAs enhance the expression of HIF-1α by competitively binding to miRNAs and relieving their inhibitory effect on HIF-1α. Circ-HIPK3 upregulates the expression of HIF-1α by adsorbing miR-338-3p, and HIF-1α mediates EMT to promote the growth and metastasis of cervical cancer cells [79]. Additionally, circ-Erbin acts as a sponge for miR-125a-5p and miR-138-5p, targeting eukaryotic translation initiation factor 4E-binding protein 1 (4EBP-1), accelerating cap-independent protein translation of HIF-1α in CRC cells, and promoting angiogenesis [80]. Moreover, CircDNMT1 targets the miR576 -3p/HIF-l α axis to promote malignant behavior and metabolic reprogramming in gastric cancer [81].
CircRNAs also participate in immune regulation under hypoxic conditions, facilitating tumor cells to evade the cytotoxic effects of immune cells by inducing M2 polarization of macrophages, suppressing T cell infiltration, and enhancing the expression of tumor PD-L1, for instance, in esophageal squamous cell carcinoma, hsa-circ-0048117 is significantly upregulated and enriched in exosomes secreted by tumor cells after hypoxia preconditioning, hsa-circ-0048117 acts as a sponge for miR-140, promoting macrophage polarization towards the M2 phenotype by competing with TLR4 [82]. In hepatocellular carcinoma under hypoxic conditions, circPRDM4 acts as a scaffold to recruit HIF-1α to the CD274 promoter and consolidates their interaction, ultimately facilitating HIF- 1α-mediated transactivation of PD-L1 while inhibiting CD8 + T-cell infiltration into the tumor microenvironment and promoting immune escape [83] (Table 3).
CircRNAs and tumor symbiotic microorganisms
Microorganisms in tumor symbiosis also exert influence on tumor growth and the remodeling of the tumor microenvironment, as they are an integral part of some tumors. A meta-analysis has revealed that approximately 15% of cancers can be directly attributed to infections caused by various etiologies such as viruses, bacteria, and parasites [84]. Furthermore, these cancers are associated with chronic inflammation, which supports the growing link between infection, inflammation, and cancer. Viral non-coding RNAs are increasingly being recognized as important regulators of infection and pathogenic mediators.
The prolonged stimulation of pathogenic bacteria results in chronic inflammation within the body, creating an immunosuppressive environment that facilitates tumor development. Tumor commensal microorganisms regulate abnormal gene expression and promote tumor progression through their own encoded circRNAs or by inducing circRNAs encoded by the tumor. Helicobacter pylori (H. pylori) is a major etiological factor in gastric cancer, and it has been shown that H. pylori infection induces circMAN1A2 expression in gastric cancer cells, promoting gastric cancer progression by acting as a sponge for miR-1236-3p and regulating MTA [85]. Additionally, H. pylori infection can increase the migration and invasion ability of gastric cancer cells by promoting the expression of circFNDC3B [86]. Hepatitis B virus (HBV) is the primary cause of hepatocellular carcinoma (HCC), and circRNAs are closely associated with HBV-induced HCC. CircBACH1 expression is elevated in both HCC tissues and HBV-transfected hepatocellular carcinoma cells, where it regulates HBV replication and hepatocellular carcinoma progression via the miR-200a-3p/MAP3K2 pathway [87]. In cervical cancer, circE7 derived from HPV16 can encode oncoprotein E7 to promote tumor growth [88]. Moreover, commensal microorganisms-encoded circRNAs also impact tumor angiogenesis. For instance, in EBV-associated gastric cancer (EBVaGC), the expression of EBV-encoded circLMP2A is positively correlated with distant metastasis and poor prognosis under hypoxic conditions due to a positive feedback loop between HIF1α and EBV-circLMP2A that promotes angiogenesis [89]. Kaposi’s sarcoma, which is commonly observed in AIDS patients, is an aggressive vascular tumor of endothelial origin caused by the oncogenic KSHV. The viral interferon regulatory factor 1 (vIRF1) encoded by KSHV induces circARFGEF1 transcription through binding to lymphoid enhancer-binding factor 1 (Lef1), thereby promoting cell motility, proliferation, and angiogenesis in vivo [90]. Merkel cell polyomavirus (MCV) expresses circRNAs, with MCV being responsible for approximately 80% of Merkel cell carcinomas (MCCs). Among these circRNAs, circMCV-T derived from MCV plays a crucial role in regulating tumor functions and viral replication [91].
Notably, the gut microbiota modulates the host’s response to cancer therapies, the composition of gut microbiota has demonstrated predictive and prognostic implications for the response to immune checkpoint blockade (ICB) therapy. The development and implementation of therapeutic strategies targeting microbial communities aim to modulate patient’s gut microbiota and its functionality, thereby optimizing clinical response to ICB treatment while minimizing treatment-related toxicity [92]. Zhu et al. demonstrated in a mouse model that SPF mice or Bifidobacterium cecum transplanted into germ-free mice significantly inhibited lung metastasis. Additionally, significant differences were observed in circRNA and miRNA expression between germ-free and SPF mice transplanted with Bifidobacterium cecum. It was found that the mmu_circ_000073/mmu-miR-466i-3p/SOX9 axis could promote EMT (epithelial-mesenchymal transition) and tumor metastasis [93]. From this, it can be seen that abnormal expression of circRNAs may be one of the reasons for tumor progression caused by dysregulated gut microbiota, and regulating the expression of circRNAs in the gut microbiota could potentially become a means of cancer treatment (Table 4).
CircRNAs and immunosuppression
As the most abundant cellular component of the TME, immune cells play pro- or anti-tumorigenic roles in the tumor microenvironment. Immune cells undergo three stages of immune editing within the TME: elimination, homeostasis, and escape, in which tumor cells evade the host’s immune response by shedding surface antigens or down-regulating the expression of key molecules required for interaction with immune cells [2, 94]. In addition, tumor cells actively recruit lymphocytes, myeloid suppressor cells, macrophages, etc. to the tumor site through the production of chemokines. Tumor-associated immune cells produce cytokines and growth factors that are essential for tumor growth but do not exert anti-tumor functions. Immunotherapy targeting immune checkpoints such as PD-1/PD-L1 as well as CTLA- 4 restores the activity of depleted CD8 + T cells to kill tumor cells, resulting in good overall survival in mutation-negative patients [95, 96]. However, not all patients derive benefit from immunotherapy and relapse is an inevitable occurrence in those receiving this treatment modality. therefore, further elucidation of the regulatory mechanisms governing immune cells within tumors remains imperative. circRNAs exert a pivotal role in regulating the functions of tumor-associated macrophages (TAMs), regulatory T (Treg) cells, CD8 + T cells, and NK cells (Table 5).
CircRNAs and macrophages
Macrophages are a heterogeneous group of immune cells that differentiate into distinct phenotypes and exhibit specific biological functions in response to various stimuli, including cytokines, growth factors, inflammation, infection, injury, hypoxia, and other conditions [97]. These cells can be classified as either classically activated M1 or alternatively activated M2 macrophages. however, the phenotypes of these subtypes can be interchanged depending on the stimulatory factors present [98]. While M1-type macrophages display pro-inflammatory effects, M2-type macrophages possess anti-inflammatory properties and promote wound healing as well as vascular-lymphangiogenic functions. Additionally, tumor-associated macrophages (TAMs), which share similarities with M2-like macrophages in terms of their phenotype and function profile, play a crucial role in promoting tumorigenesis [99].
CircRNAs exert a positive regulatory role in modulating macrophage function, and circRNAs derived from tumor cells can induce M2 polarization of macrophages [100]. Extensive studies have demonstrated that the polarization of macrophages is governed by signaling pathways such as JAK1/STAT3 and PI3K-AKT, wherein circRNAs actively participate [101,102,103,104]. For instance, in ovarian cancer, exosome-derived circATP2B4 can be delivered to infiltrating macrophages and induce M2-type polarization through modulation of the miR-532-3p/SREBF1/PI3Kα/AKT axis, leading to immunosuppression and ovarian cancer metastasis [105]. Exosomes containing circFARSA mediate M2-type polarization of macrophages through the PTEN/PI3K/AKT pathway and promote EMT and metastasis in non-small cell lung cancer [106]. In breast cancer, endoplasmic reticulum stress promotes the secretion of tumor-derived exosomes and enhances the entry of circ_0001142 into macrophages, which interferes with macrophage autophagy and polarization processes by miR-361-3p/PIK3CB axis [107]. The exosomes circSAFB2 promotes kidney cancer metastasis by mediating M2-type macrophage polarization through the miR-620/JAK1/STAT3 axis [108]. Hsa-circ-0048117 is significantly upregulated and enriched in exosomes secreted by esophageal squamous cell carcinoma after hypoxic preconditioning, and it competes with TLR4 to adsorb mir-140, promoting macrophage polarization towards the M2 phenotype [82]. It is worth noting that the phenotype of macrophages can be converted under different cytokine stimuli, inducing the transformation of tumor-associated macrophages into tumor-killing cells, which has become an important approach in immunotherapy [109], and circRNAs may serve as a potential target for this purpose.
M2 macrophages that differentiate in response to cytokines within the tumor microenvironment lose their anti-tumor efficacy and secrete immunosuppressive factors such as IL-10, TGF-β, and indoleamine 2,3-dioxygenase (IDO), thereby facilitating immune evasion [110]. Furthermore, exosomes circRNAs derived from macrophages also actively participate in various physiological processes that promote tumor growth. For instance, RBPJ + macrophage-secreted exosomes containing hsa_circ_0004658 impede the progression of hepatocellular carcinoma through the miR-499b-5p/JAM3 pathway [111]. In cutaneous squamous cell carcinoma, M2-type macrophages upregulate circ_TNFRSF21 to promote angiogenesis by competitively adsorbing miR-3619-5p and increasing ROCK expression [112]. Furthermore, TAMs-derived exosomes transfer hsa_circ_0001610 to endometrial cancer cells and enhance cyclin B1 expression by adsorbing miR-139-5p, thereby reducing the radiosensitivity of endometrial cancer cells [113]. Additionally, the exosomes circZNF451 inhibit anti-PD1 therapy in lung adenocarcinoma by polarizing macrophages in complex with TRIM56 and FXR1 [114].
CircRNAs and T lymphocytes
Tumor-infiltrating lymphocytes (TIL) are an integral part of the host’s inflammatory response to tumors. However, mounting evidence suggests that despite their activating phenotype, TIL exhibit functional impairment due to the absence of Th1 cytokines (such as IL-2, IFN-γ, and IL-12) and the prevalence of Treg cells cytokines (such as IDO or TGF-β) at tumor sites. This conversion leads to a Th2 or Treg functional phenotype in tumor-specific T-cells. Furthermore, Treg cells impede effector T cell infiltration and CD8 + T cell cytotoxicity while promoting cancer cell survival [115]. CircRNAs also play a regulatory role in tumor-infiltrating lymphocytes (TILs), as evidenced by the ability of HCC cell-derived exosomes circGSE1 to induce Treg cell expansion through modulation of the miR-324-5p/TGFBR1/Smad3 axis [116]. Hsa_circ_0136666 to regulate Treg cells activity via targeting the miR-497/PD-L1 axis in colorectal cancer [117], and cancer cell-derived exosomes circUSP7 to inhibit CD8 + T cell secretion of IFN-γ, TNF-α, granzyme-b, and perforin by adsorbing miR-934 to up-regulate protein tyrosine phosphatase 2 (SH2)-containing Src homology region 2 (SH2) expression in non-small cell lung cancer [118]. Additionally, bladder cancer cells release exosome-derived circTRPS1 that regulates intracellular reactive oxygen species homeostasis and CD8 + T cell depletion via the circTRPS1/miR141-3p/GLS1 axis [119]. It has been demonstrated that methylation modifications play a crucial role in regulating the expression, function, and stability of circRNAs [120]. Additionally, methylated circRNAs have been shown to be involved in tumor immunity regulation. In NSCLC, N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8 + T-cell responses by promoting deubiquitination of PD-L1, thereby facilitating tumor immune escape [121]. These findings provide novel insights into potential immunotherapeutic targets.
CircRNAs and NK cells
NK cells are innate immune cells that protect cells from immune attack by recognizing MHC class I molecules on the surface of normal cells. Tumor cells are recognized by NK cells due to the loss of MHC class I molecules, and NK cells play a role in tumor immunosurveillance by directly killing or releasing cytokines to clear newly arising tumors or metastases without prior sensitization. Developing tumors utilize multiple mechanisms to evade NK cell-mediated immune surveillance, resulting in limited access of NK cells to the tumor bed, altered NK cell phenotype and function, and loss of immunogenicity, which impedes recognition of tumor cells by NK cell receptors [122, 123]. The expression of immune-related molecules, such as PD-L1 and ICAM-1, is regulated by circRNAs through ceRNA networks, leading to the inhibition of NK cell activity and acceleration of their exhaustion. Consequently, this facilitates tumor immune evasion. For example, circFOXO3, hsa_circ_0048674, hsa_circ_0007456, and circRHOT1 affect the activity of NK cells by adsorbing miRNAs, causing NK cell senescence and promoting tumor growth, respectively [124,125,126,127].
Future remarks
With a comprehensive understanding of the structure and function of circRNAs, they have emerged as a crucial player in tumorigenesis, development, and regulation of the tumor microenvironment. As such, circRNAs represent an exciting area of research in cancer biology with potential implications for therapeutic interventions. By exploring the interactions between circRNAs and key components of TME, we propose that circRNAs hold promise as both diagnostic biomarkers and therapeutic targets.
References
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37.
Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8(2):98–101.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–12.
Hessmann E, Buchholz SM, Demir IE, et al. Microenvironmental determinants of pancreatic cancer. Physiol Rev. 2020;100(4):1707–51.
Ma Z, Shuai Y, Gao X, et al. Circular RNAs in the tumour microenvironment. Mol Cancer. 2020;19(1):8.
Zhang HD, Jiang LH, Sun DW, et al. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7.
Kristensen LS, Hansen TB, Veno MT, et al. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555–65.
Zhang Q, Wang W, Zhou Q, et al. Roles of circRNAs in the tumour microenvironment. Mol Cancer. 2020;19(1):14.
Liu XQ, Gao YB, Zhao LZ, et al. Biogenesis, research methods, and functions of circular RNAs. Yi Chuan. 2019;41(6):469–85.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32(5):453–61.
Liang ZZ, Guo C, Zou MM, et al. circRNA-miRNA-mRNA regulatory network in human lung cancer: an update. Cancer Cell Int. 2020;20:173.
Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8.
Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–34.
Aktas T, Avsar Ilik I, Maticzka D, et al. DHX9 suppresses RNA processing defects originating from the Alu invasion of the human genome. Nature. 2017;544(7648):115–9.
Zhang M, Huang N, Yang X, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37(13):1805–14.
Yang Y, Gao X, Zhang M, et al. Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110(3):304–15.
Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–64.
Zhang Y, Zhang XO, Chen T, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806.
Fasolo F, Di Gregoli K, Maegdefessel L, et al. Non-coding RNAs in cardiovascular cell biology and atherosclerosis. Cardiovasc Res. 2019;115(12):1732–56.
Khan AQ, Ahmad F, Raza SS, et al. Role of non-coding RNAs in the progression and resistance of cutaneous malignancies and autoimmune diseases. Semin Cancer Biol. 2022;83:208–26.
Liang Q, Fu J, Wang X, et al. circS100A11 enhances M2a macrophage activation and lung inflammation in children with asthma. Allergy. 2023;78(6):1459–72.
Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70.
Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32(35):4057–63.
Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437(7058):497–504.
Zheng X, Ma YF, Zhang XR, et al. Circ_0056618 promoted cell proliferation, migration, and angiogenesis through sponging with miR-206 and upregulating CXCR4 and VEGF-A in colorectal cancer. Eur Rev Med Pharmacol Sci. 2020;24(8):4190–202.
Jiang Y, Zhou J, Zhao J, et al. The U2AF2/circRNA ARF1/miR-342-3p/ISL2 feedback loop regulates angiogenesis in glioma stem cells. J Exp Clin Cancer Res. 2020;39(1):182.
Xie M, Yu T, Jing X, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19(1):112.
Guo Y, Guo Y, Chen C, et al. Circ3823 contributes to growth, metastasis, and angiogenesis of colorectal cancer: involvement of miR-30c-5p/TCF7 axis. Mol Cancer. 2021;20(1):93.
Xu Y, Leng K, Yao Y, et al. A circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, contributes to cholangiocarcinoma progression, induces angiogenesis, and disrupts vascular endothelial barriers. Hepatology. 2021;73(4):1419–35.
Yu L, Zhu H, Wang Z, et al. Circular RNA circFIRRE drives osteosarcoma progression and metastasis through tumorigenic-angiogenic coupling. Mol Cancer. 2022;21(1):167.
Zeng Y, Fu BM. Resistance mechanisms of anti-angiogenic therapy and exosomes-mediated revascularization in Cancer. Front Cell Dev Biol. 2020;8:610661.
Kuczynski EA, Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis. 2020;23(1):55–74.
Fathi Maroufi N, Taefehshokr S, Rashidi MR, et al. Vascular mimicry: changing the therapeutic paradigms in cancer. Mol Biol Rep. 2020;47(6):4749–65.
Shao Y, Lu B. The emerging roles of circular RNAs in vessel co-option and vasculogenic mimicry: clinical insights for anti-angiogenic therapy in cancers. Cancer Metastasis Rev. 2022;41(1):173–91.
Huang XY, Huang ZL, Huang J, et al. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 2020;39(1):20.
Ma HB, Yao YN, Yu JJ, et al. Extensive profiling of circular RNAs and the potential regulatory role of circRNA-000284 in cell proliferation and invasion of cervical cancer via sponging miR-506. Am J Transl Res. 2018;10(2):592–604.
Wang K, Sun Y, Tao W, et al. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801.
Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200.
Zhao B, Song X, Guan H. CircACAP2 promotes breast cancer proliferation and metastasis by targeting miR-29a/b-3p-COL5A1 axis. Life Sci. 2020;244:117179.
Huang P, Li M, Tang Q, et al. Circ_0000523 regulates miR-1184/COL1A1/PI3K/Akt pathway to promote nasopharyngeal carcinoma progression. Apoptosis. 2022;27(9–10):751–61.
Zhang J, Peng Y, Jiang S, et al. Hsa_circRNA_0001971 contributes to oral squamous cell carcinoma progression via miR-186-5p/Fibronectin type III domain containing 3B axis. J Clin Lab Anal. 2022;36(3):e24245.
Luo G, Li R, Li Z. CircRNA circFNDC3B promotes esophageal cancer progression via cell proliferation, apoptosis, and migration regulation. Int J Clin Exp Pathol. 2018;11(8):4188–96.
Hong Y, Qin H, Li Y, et al. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E-cadherin and CD44 expression. J Cell Physiol. 2019;234(11):19895–910.
Pan Z, Cai J, Lin J, et al. A novel protein encoded by circFNDC3B inhibits tumor progression and EMT through regulating snail in colon cancer. Mol Cancer. 2020;19(1):71.
Wang X, Tan M, Huang H, et al. Hsa_circ_0000285 contributes to gastric cancer progression by upregulating FN1 through the inhibition of miR-1278. J Clin Lab Anal. 2022;36(6):e24475.
Du J, Zhang G, Qiu H, et al. The novel circular RNA circ-CAMK2A enhances lung adenocarcinoma metastasis by regulating the miR-615-5p/fibronectin 1 pathway. Cell Mol Biol Lett. 2019;24:72.
Couchman JR. Syndecan-1 (CD138), carcinomas and EMT. Int J Mol Sci. 2021;22(8):4227.
Onyeisi JOS, Lopes CC, Gotte M. Syndecan-4 as a pathogenesis factor and therapeutic target in cancer. Biomolecules. 2021;11(4):503.
Rapraeger AC. Syndecan-regulated receptor signaling. J Cell Biol. 2000;149(5):995–8.
Chen W, Yu X, Wang N, et al. Circ_RPPH1 regulates glioma cell malignancy by binding to miR-627-5p/miR-663a to induce SDC1 expression. Metab Brain Dis. 2022;37(4):1231–45.
Tang M, Wang F, Wang K, et al. Circ_0058063 promotes progression of thyroid cancer by sponging miR-330-3p/SDC4 axis. Anticancer Drugs. 2022;33(7):642–51.
Spinelli FM, Vitale DL, Sevic I, et al. Hyaluronan in the tumor microenvironment. Adv Exp Med Biol. 2020;1245:67–83.
Kim YH, Lee SB, Shim S, et al. Hyaluronic acid synthase 2 promotes malignant phenotypes of colorectal cancer cells through transforming growth factor beta signaling. Cancer Sci. 2019;110(7):2226–36.
Karousou E, Misra S, Ghatak S, et al. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017;59:3–22.
Zhang W, Liu T, Li T, et al. Hsa_circRNA_102002 facilitates metastasis of papillary thyroid cancer through regulating miR-488-3p/HAS2 axis. Cancer Gene Ther. 2021;28(3–4):279–93.
Dai W, Zhai X, Chen Y, et al. CircMMP1 promotes colorectal cancer growth and metastasis by sponging miR-1238 and upregulating MMP family expression. Ann Transl Med. 2021;9(16):1341.
Huang F, Jiang J, Yao Y, et al. Circular RNA Hsa_circRNA_101996 promotes the development of gastric Cancer via Upregulating Matrix Metalloproteinases-2/Matrix Metalloproteinases-9 through MicroRNA-143/Ten-eleven translocation-2 Pathway. J Cancer. 2021;12(22):6665–75.
Sang M, Meng L, Liu S, et al. Circular RNA ciRS-7 maintains metastatic phenotypes as a ceRNA of miR-1299 to Target MMPs. Mol Cancer Res. 2018;16(11):1665–75.
Tsoumakidou M. The advent of immune stimulating CAFs in cancer. Nat Rev Cancer. 2023;23(4):258–69.
Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer. 2021;20(1):131.
Geng X, Chen H, Zhao L, et al. Cancer-associated fibroblast (CAF) heterogeneity and targeting therapy of CAFs in pancreatic cancer. Front Cell Dev Biol. 2021;9:655152.
Zheng S, Hu C, Lin H, et al. circCUL2 induces an inflammatory CAF phenotype in pancreatic ductal adenocarcinoma via the activation of the MyD88-dependent NF-kappaB signaling pathway. J Exp Clin Cancer Res. 2022;41(1):71.
Liu G, Sun J, Yang Z-F, et al. Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 2021;12(3):260.
Zhou Y, Tang W, Zhuo H, et al. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/ nuclear factor -kappa B (NF-kappaB) pathway. Bioengineered. 2022;13(3):4786–97.
Hu C, Xia R, Zhang X, et al. circFARP1 enables cancer-associated fibroblasts to promote gemcitabine resistance in pancreatic cancer via the LIF/STAT3 axis. Mol Cancer. 2022;21(1):1–21.
Shao C, Yang F, Miao S, et al. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17(1):120.
Jing X, Yang F, Shao C, et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157.
Riera-Domingo C, Audige A, Granja S, et al. Immunity, hypoxia, and metabolism-the menage a trois of cancer: implications for immunotherapy. Physiol Rev. 2020;100(1):1–102.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Li Q, Pan X, Zhu D, et al. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70(4):1298–316.
Jiang X, Guo S, Wang S, et al. EIF4A3-Induced circARHGAP29 promotes aerobic glycolysis in docetaxel-resistant prostate cancer through IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 2022;82(5):831–45.
Lin J, Wang X, Zhai S, et al. Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. J Hematol Oncol. 2022;15(1):128.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96.
Tirpe AA, Gulei D, Ciortea SM, et al. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci. 2019;20(24):6140.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–32.
Qian W, Huang T, Feng W, Circular. RNA HIPK3 promotes EMT of cervical cancer through sponging mir-338-3p to up-regulate HIF-1alpha. Cancer Manag Res. 2020;12:177–87.
Chen LY, Wang L, Ren YX, et al. The circular RNA circ-ERBIN promotes growth and metastasis of colorectal cancer by miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1alpha translation. Mol Cancer. 2020;19(1):164.
Li H, Cao B, Zhao R, et al. circDNMT1 promotes malignant progression of gastric cancer through targeting miR-576-3p/Hypoxia inducible factor-1 alpha axis. Front Oncol. 2022;12:817192.
Lu Q, Wang X, Zhu J, et al. Hypoxic tumor-derived exosomal Circ0048117 facilitates M2 macrophage polarization acting as miR-140 sponge in esophageal squamous cell carcinoma. Onco Targets Ther. 2020;13:11883–97.
Chen ZQ, Zuo XL, Cai J, et al. Hypoxia-associated circPRDM4 promotes immune escape via HIF-1alpha regulation of PD-L1 in hepatocellular carcinoma. Exp Hematol Oncol. 2023;12(1):17.
De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–15.
Guo R, Cui X, Li X, et al. CircMAN1A2 is upregulated by helicobacter pylori and promotes development of gastric cancer. Cell Death Dis. 2022;13(4):409.
Zhang J, Bai J, Zhu H, et al. The upregulation of circFNDC3B aggravates the recurrence after endoscopic submucosal dissection (ESD) in early gastric cancer (EGC) patients. Sci Rep. 2022;12(1):6178.
Du N, Li K, Wang Y, et al. CircRNA circBACH1 facilitates hepatitis B virus replication and hepatoma development by regulating the miR-200a-3p/MAP3K2 axis. Histol Histopathol. 2022;37(9):863–77.
Zhao J, Lee EE, Kim J, et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10(1):2300.
Du Y, Zhang JY, Feng ZY, et al. Hypoxia-induced ebv-circLMP2A promotes angiogenesis in EBV-associated gastric carcinoma through the KHSRP/VHL/HIF1 alpha/VEGFA pathway. Cancer Lett. 2022;526:259–72.
Yao S, Jia X, Wang F, et al. CircRNA ARFGEF1 functions as a ceRNA to promote oncogenic KSHV-encoded viral interferon regulatory factor induction of cell invasion and angiogenesis by upregulating glutaredoxin 3. PLoS Pathog. 2021;17(2):e1009294.
Abere B, Zhou H, Li J, et al. Merkel cell polyomavirus encodes circular RNAs (circRNAs) enabling a dynamic circRNA/microRNA/mRNA regulatory network. mBio. 2020;11(6).
Park EM, Chelvanambi M, Bhutiani N, et al. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28(4):690–703.
Zhu Z, Huang J, Li X, et al. Gut microbiota regulate tumor metastasis via circRNA/miRNA networks. Gut Microbes. 2020;12(1):1788891.
Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25.
Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81.
Pitt JM, Vetizou M, Daillere R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–69.
Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55.
Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119(3):414–7.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Entezari M, Sadrkhanloo M, Rashidi M, et al. Non-coding RNAs and macrophage interaction in tumor progression. Crit Rev Oncol Hematol. 2022;173:103680.
Yang T, Wang R, Liu H, et al. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021;266:118903.
Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954.
Zhao HY, Zhang YY, Xing T, et al. M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity. Signal Transduct Target Ther. 2021;6(1):234.
Zhao SJ, Kong FQ, Jie J, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3beta/beta-catenin pathway. Theranostics. 2020;10(1):17–35.
Wang F, Niu Y, Chen K, et al. Extracellular vesicle-packaged circATP2B4 mediates M2 macrophage polarization via miR-532-3p/SREBF1 Axis to promote epithelial ovarian cancer metastasis. Cancer Immunol Res. 2023;11(2):199–216.
Chen T, Liu Y, Li C, et al. Tumor-derived exosomal circFARSA mediates M2 macrophage polarization via the PTEN/PI3K/AKT pathway to promote non-small cell lung cancer metastasis. Cancer Treat Res Commun. 2021;28:100412.
Lu C, Shi W, Hu W, et al. Endoplasmic reticulum stress promotes breast cancer cells to release exosomes circ_0001142 and induces M2 polarization of macrophages to regulate tumor progression. Pharmacol Res. 2022;177:106098.
Huang X, Wang J, Guan J, et al. Exosomal Circsafb2 reshaping tumor environment to promote renal cell carcinoma progression by mediating M2 macrophage polarization. Front Oncol. 2022;12:808888.
Mantovani A, Allavena P, Marchesi F, et al. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21(11):799–820.
Vitale I, Manic G, Coussens LM, et al. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50.
Zhang L, Zhang J, Li P, et al. Exosomal hsa_circ_0004658 derived from RBPJ overexpressed-macrophages inhibits hepatocellular carcinoma progression via miR-499b-5p/JAM3. Cell Death Dis. 2022;13(1):32.
Ma J, Huang L, Gao YB, et al. M2 macrophage facilitated angiogenesis in cutaneous squamous cell carcinoma via circ_TNFRSF21/miR-3619-5p/ROCK axis. Kaohsiung J Med Sci. 2022;38(8):761–71.
Gu X, Shi Y, Dong M, et al. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021;12(9):818.
Gao J, Ao YQ, Zhang LX, et al. Exosomal circZNF451 restrains anti-PD1 treatment in lung adenocarcinoma via polarizing macrophages by complexing with TRIM56 and FXR1. J Exp Clin Cancer Res. 2022;41(1):295.
Brown CC, Gottschalk RA. Volume control: turning the dial on regulatory T cells. Cell. 2021;184(15):3847–9.
Huang M, Huang X, Huang N. Exosomal circGSE1 promotes immune escape of hepatocellular carcinoma by inducing the expansion of regulatory T cells. Cancer Sci. 2022;113(6):1968–83.
Xu YJ, Zhao JM, Gao C, et al. Hsa_circ_0136666 activates treg-mediated immune escape of colorectal cancer via miR-497/PD-L1 pathway. Cell Signal. 2021;86:110095.
Chen SW, Zhu SQ, Pei X, et al. Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer. 2021;20(1):144.
Yang C, Wu S, Mou Z, et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8 + T cell exhaustion in bladder cancer microenvironments. Mol Ther. 2022;30(3):1054–70.
Wang X, Ma R, Zhang X, et al. Crosstalk between N6-methyladenosine modification and circular RNAs: current understanding and future directions. Mol Cancer. 2021;20(1):121.
Liu Z, Wang T, She Y, et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. 2021;20(1):105.
Song-Yang Wu TF. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:26.
Cozar B, Greppi M, Carpentier S, et al. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11(1):34–44.
Yang F, Chen Y, Luo L, et al. circFOXO3 Induced by KLF16 modulates clear cell renal cell carcinoma growth and natural killer cell cytotoxic activity through sponging miR-29a-3p and miR-122-5p. Dis Markers. 2022;2022:6062236.
Li S, Chen Z, Zhou R, et al. Hsa_circ_0048674 facilitates hepatocellular carcinoma progression and natural killer cell exhaustion depending on the regulation of miR-223-3p/PDL1[J]. Histol Histopathol. 2022;37(12):1185–99.
Shi M, Li ZY, Zhang LM, et al. Hsa_circ_0007456 regulates the natural killer cell-mediated cytotoxicity toward hepatocellular carcinoma via the miR-6852-3p/ICAM-1 axis[J]. Cell Death Dis. 2021;12(1):94.
Ke H, Zhang J, Wang F, et al. ZNF652-Induced circRHOT1 Promotes SMAD5 expression to modulate tumorigenic properties and nature killer cell-mediated toxicity in bladder cancer via targeting miR-3666. J Immunol Res. 2021;2021:7608178.
Funding
This study was supported by the Natural Science and Technology Fund of Guizhou Province (grant no.: Qiankehe Basic-ZK [2022] General 644, Qiankehe support-ZK [2021] General 081 and Qiankehe support-ZK [2021] General 082).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The literature search was performed by TL, KL, and ZZ. The first draft of the manuscript was written by TL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.