Abstract
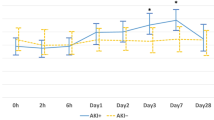
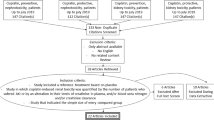
Cisplatin is used to treat solid malignancies including head and neck cancer. However, nephrotoxicity limits its use. In this study, we looked for a possible protective effect of pantoprazole against cisplatin-induced nephrotoxicity. We used novel biomarkers for early detection of nephrotoxicity. Sixty chemotherapy naïve head and neck cancer patients completed the study. Following complete history taking and thorough clinical examination, patients were randomly divided into three groups: 20 patients in each. Group I (control group) received cisplatin without pantoprazole, groups II and III received pantoprazole 80 mg and 40 mg, respectively, concurrently with cisplatin. Blood and urine samples were collected at baseline, and 48 h after the first and third cycles of cisplatin administration. Assessment of serum creatinine and soluble FasL (sFasL), as well as urinary neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) was performed. Nephrotoxicity was detected in 6 patients in group I, none in group II and 3 patients in group III. Serum creatinine significantly increased at the end of treatment in group I compared to groups II and III. Group I also had significantly higher urinary KIM-1 and NGAL and serum sFasL compared to groups II and III after the first and third cycles. On the contrary, there was no significant difference between groups II and III. Pantoprazole prevented the increase in acute kidney injury biomarkers in cisplatin-treated patients. Therefore, it is a promising agent in reducing cisplatin-induced nephrotoxicity.
Trial registration Clinical Trials.gov identifier: NCT04217512, registered in January 2020 " retrospectively registered".




Similar content being viewed by others
Data availability
All data and materials are transparent, support published claims and comply with field standards.
References
Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. https://doi.org/10.1007/s40620-017-0392-z.
Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel). 2010;2:2490–518. https://doi.org/10.3390/toxins2112490.
Ozkok A, Edelstein CL. Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int. 2014. https://doi.org/10.1155/2014/967826.
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. https://doi.org/10.1038/sj.ki.5002786.
Peres LAB, da Cunha AD. Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol ʹorgão Of Soc Bras e Latino-Americana Nefrol. 2013;35:332–40. https://doi.org/10.5935/0101-2800.20130052.
Duan ZY, Cai GY, Li JJ, Chen XM. Cisplatin-induced renal toxicity in elderly people. Ther Adv Med Oncol. 2020;12:1–15. https://doi.org/10.1177/1758835920923430.
Tanase DM, Gosav EM, Radu S, Costea CF, Ciocoiu M, Carauleanu A, et al. The predictive role of the biomarker kidney molecule-1 (KIM-1) in acute kidney injury (AKI) cisplatin-induced nephrotoxicity. Int J Mol Sci. 2019;20:5238. https://doi.org/10.3390/ijms20205238.
Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010;176:1169–80. https://doi.org/10.2353/ajpath.2010.090610.
Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, et al. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–84. https://doi.org/10.1016/S0002-9440(10)61234-5.
Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014;20:4026–35. https://doi.org/10.1158/1078-0432.CCR-14-0319.
Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. https://doi.org/10.1038/clpt.2009.139.
Seijas M, Baccino C, Nin N, Lorente JA. Definition and biomarkers of acute renal damage: new perspectives. Med Intensiva. 2014;38:376–85. https://doi.org/10.1016/j.medine.2013.09.003.
Ghadrdan E, Ebrahimpour S, Sadighi S, Chaibakhsh S, Jahangard-Rafsanjani Z. Evaluation of urinary neutrophil gelatinase-associated lipocalin and urinary kidney injury molecule-1 as biomarkers of renal function in cancer patients treated with cisplatin. J Oncol Pharm Pract. 2020;26(7):1643–9. https://doi.org/10.1177/1078155220901756.
Kubrak T, Podgórski R, Aebisher D, Gala-Błądzińska A. The significance of NGAL and KIM-1 proteins for diagnosis of acute kidney injury (AKI) in clinical practice. Eur J Clin Exp Med 2018;16:28-33. https://doi.org/10.15584/ejcem.2018.1.4.
Ajay M, G R, Gottipati SS, Babu P S. A concise review on extensive use of proton pump inhibitors. Transl Med 2018;08:8–10. https://doi.org/10.4172/2161-1025.1000204.
Strand DS, Kim D, Peura DA. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11:27–37. https://doi.org/10.5009/gnl15502.
Nies AT, Hofmann U, Resch C, Schaeffeler E, Rius M, Schwab M. Proton pump inhibitors inhibit metformin uptake by organic cation transporters (OCTs). PLoS ONE. 2011;6(7): e22163. https://doi.org/10.1371/journal.pone.0022163.
Cancer Therapy Evaluation Program (CTEP). Common Terminology Criteria for Adverse Events (CTCAE).v.5.0 [5x7]. Cancer Ther Eval Progr 2017:155. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. Accessed 5 Feb 2021.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. https://doi.org/10.1159/000180580.
Snchez-Gonzlez PD, López-Hernández FJ, López-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol. 2011;41:803–21. https://doi.org/10.3109/10408444.2011.602662.
Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26:1–14. https://doi.org/10.1186/s12929-019-0518-9.
Bunel V, Tournay Y, Baudoux T, De Prez E, Marchand M, Mekinda Z, et al. Early detection of acute cisplatin nephrotoxicity: interest of urinary monitoring of proximal tubular biomarkers. Clin Kidney J. 2017;10:639–47. https://doi.org/10.1093/ckj/sfx007.
Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M, Abdollahi A, et al. Effect of silymarin administration on cisplatin nephrotoxicity: report from a pilot, randomized, double-blinded, Placebo-controlled clinical. Trial Phyther Res. 2015;29:1046–53. https://doi.org/10.1002/ptr.5345.
Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: State of the art and recommendations from the european society of clinical pharmacy special interest group on cancer care. Cancer Chemother Pharmacol. 2008;61:903–9. https://doi.org/10.1007/s00280-008-0711-0.
Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–27. https://doi.org/10.1016/j.toxlet.2015.06.012.
Schaeffeler E, Hellerbrand C, Nies AT, Winter S, Kruck S, Hofmann U, van der Kuip H, Zanger UM, Koepsell HSM. DNA methylation is associated with downregulation of the organic cation transporter OCT1 (SLC22A1) in human hepatocellular carcinoma. Genome Med. 2011;3:82. https://doi.org/10.1186/gm298.
Chen L, Hong C, Chen EC, Yee SW, Xu L, Almof EU, et al. Genetic and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharmacogenomics J. 2013;13:110–20. https://doi.org/10.1038/tpj.2011.60.
Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014;550:547–50. https://doi.org/10.6064/2012/473829.
Shariatmaghani SS, Saadat A, Nazar I, Davoudi F, Parvin S, Mehrani H, et al. Urinary neutrophil gelatinase associated lipocalin (NGAL) in predicting Cisplatin-Induced acute kidney injury. Nephrourol Mon. 2019;11: e87523. https://doi.org/10.5812/numonthly.87523.
Tezcan S, Izzettin FV, Sancar M, Yumuk PF, Turhal S. Nephrotoxicity evaluation in outpatients treated with cisplatin-based chemotherapy using a short hydration method. Pharmacol & Pharm. 2013;04:296–302. https://doi.org/10.4236/pp.2013.43043.
Peres LAB, da Cunha AD, Assumpção RAB, Schäfer A, da Silva AL, Gaspar AD, et al. Evaluation of the cisplatin nephrotoxicity using the urinary neutrophil gelatinase-associated lipocalin (NGAL) in patients with head and neck cancer. J Bras Nefrol. 2014;36:280–8. https://doi.org/10.5935/0101-2800.20140041.
George B, Wen X, Mercke N, Gomez M, O’Bryant C, Bowles DW, et al. Profiling of kidney injury biomarkers in patients receiving cisplatin: time-dependent changes in the absence of clinical nephrotoxicity. Clin Pharmacol Ther. 2017;101:510–8. https://doi.org/10.1002/cpt.606.
Maeda A, Ando H, Ura T, Muro K, Aoki M, Saito K, et al. Differences in urinary renal failure biomarkers in cancer patients initially treated with cisplatin. Anticancer Res 2017;37:5235–5239. https://doi.org/10.21873/anticanres.11947.
Shahbazi F, Sadighi S, Dashti-Khavidaki S, Shahi F, Mirzania M. Urine ratio of neutrophil gelatinase-associated lipocalin to creatinine as a marker for early detection of cisplatinassociated nephrotoxicity. Iran J Kidney Dis. 2015;9:305–10.
Lin HYH, Lee SC, Lin SF, Hsiao HH, Liu YC, Yang WC, et al. Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J Med Sci. 2013;29:304–11. https://doi.org/10.1016/j.kjms.2012.10.004.
Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, Sato T, et al. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol. 2015;76:989–96. https://doi.org/10.1007/s00280-015-2880-y.
Karademir LD, Dogruel F, Kocyigit I, Yazici C, Unal A, Sipahioglu MH, et al. The efficacy of theophylline in preventing cisplatin-related nephrotoxicity in patients with cancer. Ren Fail. 2016;38:806–14. https://doi.org/10.3109/0886022X.2016.1163154.
Linkermann A, Himmerkus N, Rölver L, Keyser KA, Steen P, Bräsen JH, et al. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. 2011;79:169–78. https://doi.org/10.1038/ki.2010.317.
Soni H, Kaminski D, Gangaraju R, Adebiyi A. Cisplatin-induced oxidative stress stimulates renal fas ligand shedding. Ren Fail. 2018;40:314–22. https://doi.org/10.1080/0886022X.2018.1456938.
Ikemura K, Oshima K, Enokiya T, Okamoto A, Oda H, Mizuno T, et al. Co-administration of proton pump inhibitors ameliorates nephrotoxicity in patients receiving chemotherapy with cisplatin and fluorouracil: a retrospective cohort study. Cancer Chemother Pharmacol. 2017;79:943–9. https://doi.org/10.1007/s00280-017-3296-7.
Ismail RS, El-Awady MS, Hassan MH. Pantoprazole abrogated cisplatin-induced nephrotoxicity in mice via suppression of inflammation, apoptosis, and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1161–71. https://doi.org/10.1007/s00210-020-01823-3.
Hiramatsu S ichi, Ikemura K, Fujisawa Y, Iwamoto T, Okuda M. Concomitant lansoprazole ameliorates cisplatin-induced nephrotoxicity by inhibiting renal organic cation transporter 2 in rats. Biopharm Drug Dispos 2020;41:239–247. https://doi.org/10.1002/bdd.2242.
Fox E, Levin K, Zhu Y, Segers B, Balamuth N, Womer R, et al. Pantoprazole, an inhibitor of the organic cation transporter 2, does not ameliorate cisplatin-related ototoxicity or nephrotoxicity in children and adolescents with newly diagnosed osteosarcoma treated with methotrexate, doxorubicin, and cisplatin. Oncologist. 2018;23:762. https://doi.org/10.1634/theoncologist.2018-0037.
Carron PL, Padilla M, Maurizi BJ. Nephrotic syndrome and acute renal failure during pegylated liposomal doxorubicin treatment. Hemodial Int. 2014;18:846–7. https://doi.org/10.1111/hdi.12196.
Mohamed N, Goldstein J, Schiff J, John R. Collapsing glomerulopathy following anthracycline therapy. Am J Kidney Dis. 2013;61:778–81. https://doi.org/10.1053/j.ajkd.2012.08.048.
Acknowledgements
The authors are grateful for physicians of Oncology and Nuclear Medicine department at Menoufia University hospital for their help during patient allocation. We are also grateful for Faculty of Pharmacy, Tanta University for encouraging this research.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Patient allocation, data collection and analysis were performed by EG and SG. The first draft of the manuscript was written by EG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study protocol was approved by the Research Ethics Committee of Tanta University and was carried out in compliance with the Declaration of Helsinki.
Consent to participate
All participants provided an informed consent before being enrolled in this study.
Consent for publication
Not applicable as no personal data is included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghonaim, E., El-Haggar, S. & Gohar, S. Possible protective effect of pantoprazole against cisplatin-induced nephrotoxicity in head and neck cancer patients: a randomized controlled trial. Med Oncol 38, 108 (2021). https://doi.org/10.1007/s12032-021-01558-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01558-y