Abstract
Purpose
Cisplatin is a chemotherapeutic agent with potential nephrotoxicity. Delayed increase in serum creatinine after cisplatin administration shows that serum creatinine may not be a sufficient marker for early detection of nephrotoxicity. Urinary insulin-like growth factor binding protein-7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) which are cell-cycle arrest biomarkers have been proposed recently for early detection of acute kidney injury (AKI). Herein, we evaluated urinary TIMP-2/IGFBP7 levels before and after cisplatin administration to patients with lung cancer and their role was examined in the early diagnosis of AKI.
Methods
Patients with glomerular filtration rate above 60 mL/min who had cisplatin treatment were enrolled. Urinary TIMP-2/IGFBP7 and serum creatinine levels were measured before and at 24th hour after cisplatin administration. Serum creatinine level was also measured at 48th hour after treatment.
Results
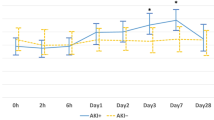
Cisplatin-associated AKI was detected in 13 patients (28%) among the 45 patients enrolled. There was no difference between creatinine, IGFBP7 and (IGFBP7 × TIMP-2)/1000 levels before and after treatment; urinary TIMP-2 level at 24th hour was significantly higher than the level before the treatment (p = 0.02). (IGFBP7 × TIMP-2)/1000 values were not different between patients with or without AKI. The area under the curve of (IGFBP7 × TIMP-2)/1000 at 24th hour of the treatment was 0.46 (CI 0.26–0.67).
Conclusion
Although urinary IGFBP7 and TIMP-2 levels are used as biomarkers for early detection of AKI for patients in intensive care units and after surgery, they seem not to be useful for early detection of AKI due to cisplatin.


Similar content being viewed by others
References
Lebwohl D, Canetta R (1998) Clinical development of platinum complexes in cancer therapy: an historical perspective and an update. Eur J Cancer 34(10):1522–1534
Cooley M, Davis LE, Stefano M, Abraham J (1994) Cisplatin: a clinical review. Part 1—current uses of cisplatin and administration guidelines. Cancer Nurs 17(3):173–184
Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M, European Society of Clinical Pharmacy Special Interest Group on Cancer Care (2008) Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol 61(6):903–909
Price PM, Safirstein RL, Megyesi J (2009) The cell cycle and acute kidney injury. Kidney Int 76(6):604–613
Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T, Wei B, Han JW, Stetler-Stevenson WG (2006) Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem 281(6):3711–3721
Cornelison TL, Reed E (1993) Nephrotoxicity and hydration management for cisplatin, carboplatin and ormaplatin. Gynecol Oncol 50(2):147–158
Yao X, Panichpisal K, Kurtzman N, Nugent K (2007) Cisplatin nephrotoxicity: a review. Am J Med Sci 334(2):115–124
Gaspari F, Cravedi P, Mandalà M, Perico N, de Leon FR, Stucchi N, Ferrari S, Labianca R, Remuzzi G, Ruggenenti P (2010) Predicting cisplatin-induced acute kidneyinjury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case–control study. Nephron Clin Pract 115(2):c154–c160
Lin HY, Lee SC, Lin SF, Hsiao HH, Liu YC, Yang WC, Hwang DY, Hung CC, Chen HC, Guh JY (2013) Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J Med Sci 29(6):304–311
Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, Sato T, Kim YH, Mishima M, Ichimura T, Bonventre JV, Matsubara K (2015) Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol 76(5):989–996
Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL (2007) Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322(1):8–15
Rodier F, Campisi J, Bhaumik D (2007) Two faces of p53: aging and tumor suppression. Nucleic Acids Res 35:7475–7484
Kashani K, Al-Khafaji A, Ardiles T et al (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17(1):R25
Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA (2015) Sapphire Investigators: tissue Inhibitor Metalloproteinase-2(TIMP-2)·IGF-Binding Protein-7 (IGFBP7) Levels Are Associated with Adverse Long-Term Outcomes in Patients with AKI. J Am Soc Nephrol 26(7):1747–1754
Bihorac A, Chawla LS, Shaw AD et al (2014) Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 189(8):932–939
Hoste EA, McCullough PA, Kashani K et al (2014) Sapphire Investigators: derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transpl 29(11):2054–2061
Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A (2014) Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 9(3):e93460
Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, Nerlich M, Schlitt HJ, Kellum JA, Bein T (2015) Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS ONE 10(3):e0120863
Kimmel M, Shi J, Latus J, Wasser C, Kitterer D, Braun N, Alscher MD (2016) Association of renal stress/damage and filtration biomarkers with subsequent AKI during hospitalization among patients presenting to the emergency department. Clin J Am Soc Nephrol 11(6):938–946
Bell M, Larsson A, Venge P, Bellomo R, Mårtensson J (2015) Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Mark 2015:158658
Prof. Dr. İrfan Şencan, Prof. Dr. Gülsün Nurhan İnce (2016) Türkiye Kanser İstatistikleri. http://kanser.gov.tr/Dosya/ca_istatistik/ANA_rapor_2013v01_2.pdf
Acknowledgements
The research received no specific grant from any funding agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the research ethics board of Okmeydani Training and Research Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Toprak, Z., Cebeci, E., Helvaci, S.A. et al. Cisplatin nephrotoxicity is not detected by urinary cell-cycle arrest biomarkers in lung cancer patients. Int Urol Nephrol 49, 1041–1047 (2017). https://doi.org/10.1007/s11255-017-1556-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1556-4