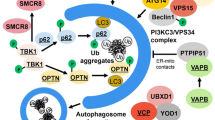
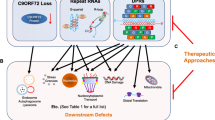
Abstract
Over the last couple of decades, there has been a growing body of clinical, genetic, and histopathological evidence that similar pathological processes underlie amyotrophic lateral sclerosis (ALS) and some types of frontotemporal lobe dementia (FTD). Even though there is great diversity in the genetic causes of these disorders, there is a high degree of overlap in their histopathology. Genes linked to rare cases of familial ALS and/or FTD, like FUS, TARDBP, OPTN, and UBQLN2 may converge onto a unifying pathogenic pathway and thereby provide novel therapeutic targets common to a spectrum of etiologically diverse forms of ALS and ALS–FTD. Additionally, there are major loci for ALS–FTD on chromosomes 9p and 15q. Identification of causative genetic alterations at those loci will be an important step in understanding the pathogenesis of juvenile- and adult-onset ALS and ALS–FTD. Interactions between TDP-43, FUS, optineurin, and ubiquilin 2 need to be studied to understand their common molecular pathways. Future efforts should also be directed towards generation and characterization of in vivo models to dissect the pathogenic mechanisms of these diseases. Such efforts will rapidly accelerate the discovery of new drugs that regulate accumulation of pathogenic proteins and their downstream consequences.




Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- FALS:
-
Familial amyotrophic lateral sclerosis
- SALS:
-
Sporadic amyotrophic lateral sclerosis
- JALS:
-
Juvenile-onset amyotrophic lateral sclerosis
- FTD:
-
Frontotemporal lobe dementia
- ALS–FTD:
-
Amyotrophic lateral sclerosis with frontotemporal lobe dementia
- PDB:
-
Paget disease of the bone
- SNP:
-
Single nucleotide polymorphism
- GWAS:
-
Genome-wide association study
- FTLD:
-
Frontotemporal lobar degeneration
- FTLD-U:
-
Frontotemporal lobe dementia with tau-negative, ubiquitin-positive inclusions
- FTLD–TDP:
-
Frontotemporal lobe dementia with ubiquitin-positive, TDP-43-positive inclusions
- FTLD–FUS:
-
Frontotemporal lobe dementia with ubiquitin-positive, FUS-positive inclusions
- SOD1:
-
Cu/Zn superoxide dismutase
- TARDBP:
-
TAR DNA binding protein
- FUS:
-
Fused in sarcoma
- OPTN:
-
Optineurin
- SQSTM1:
-
Sequestosome 1
- UBQLN2:
-
Ubiquilin 2
- VCP:
-
Valosin-containing protein
References
Albagha OM, Visconti MR, Alonso N et al (2010) Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat Genet 42:520–524
Annesi G, Savettieri G, Pugliese P et al (2005) DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann Neurol 58:803–807
Ash PE, Zhang YJ, Roberts CM et al (2010) Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet 19:3206–3218
Baumer D, Hilton D, Paine SM et al (2010) Juvenile ALS with basophilic inclusions is a FUS proteinopathy with FUS mutations. Neurology 75:611–618
Ben Hamida M, Hentati F, Ben Hamida C (1990) Hereditary motor system diseases (chronic juvenile amyotrophic lateral sclerosis). Conditions combining a bilateral pyramidal syndrome with limb and bulbar amyotrophy. Brain 113(Pt 2):347–363
Benajiba L, Le Ber I, Camuzat A et al (2009) TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol 65:470–473
Blair IP, Williams KL, Warraich ST et al (2010) FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry 81:639–645
Borroni B, Bonvicini C, Alberici A et al (2009) Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat 30:E974–E983
Boxer AL, Mackenzie IR, Boeve BF et al (2011) Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry 82:196–203
Butterfield RJ, Ramachandran D, Hasstedt SJ et al (2009) A novel form of juvenile recessive ALS maps to loci on 6p25 and 21q22. Neuromuscul Disord 19:279–287
Chen YZ, Bennett CL, Huynh HM et al (2004) DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet 74:1128–1135
Chio A, Calvo A, Moglia C et al (2010) Amyotrophic lateral sclerosis-frontotemporal lobar dementia in 3 families with p.Ala382Thr TARDBP mutations. Arch Neurol 67:1002–1009
Chow CY, Landers JE, Bergren SK et al (2009) Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet 84:85–88
Cox LE, Ferraiuolo L, Goodall EF et al (2010) Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS). PLoS One 5:e9872
Deng HX, Hentati A, Tainer JA et al (1993) Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 261:1047–1051
Deng HX, Zhai H, Bigio EH et al (2010) FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol 67:739–748
Deng H-X, Bigio E, Zhai H et al (2011a) Differential involvement of optineurin in amyotrophic lateral sclerosis with or without SOD1 mutations. Arch Neurol 68(8):1057–1061
Deng HX, Chen W, Hong S-T et al (2011b) Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature, in press
Emery AE, Holloway S (1982) Familial motor neuron diseases. Adv Neurol 36:139–147
Fecto F, Deng H-X, Siddique T (2010) Discovering the connection between familial and sporadic amyotrophic lateral sclerosis: pathology trumps genetics. Future Neurol 5:625–628
Fecto F, Yan J, Vemula SP et al (2011) SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol, in press
Finlayson MH, Martin JB (1973) Cerebral lesions in familial amyotrophic lateral sclerosis and dementia. Acta Neuropathol 26:237–246
Gouveia LO, de Carvalho M (2007) Young-onset sporadic amyotrophic lateral sclerosis: a distinct nosological entity? Amyotroph Lateral Scler 8:323–327
Greenway MJ, Andersen PM, Russ C et al (2006) ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet 38:411–413
Gurney ME, Pu H, Chiu AY et al (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264:1772–1775
Hasegawa M, Arai T, Nonaka T et al (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Hayward C, Brock DJ, Minns RA, Swingler RJ (1998) Homozygosity for Asn86Ser mutation in the CuZn-superoxide dismutase gene produces a severe clinical phenotype in a juvenile onset case of familial amyotrophic lateral sclerosis. J Med Genet 35:174
Hentati A, Bejaoui K, Pericak-Vance MA et al (1994) Linkage of recessive familial amyotrophic lateral sclerosis to chromosome 2q33-q35. Nat Genet 7:425–428
Hentati A, Ouahchi K, Pericak-Vance MA et al (1998) Linkage of a commoner form of recessive amyotrophic lateral sclerosis to chromosome 15q15-q22 markers. Neurogenetics 2:55–60
Hicks GG, Singh N, Nashabi A et al (2000) Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nat Genet 24:175–179
Hoffmann J (1894) Ueber einen eigenartigen Symptomencomplex, eine Combination von angeborenem Schwachsinn mit progressiver Muskelatrophie, als weiteren Beitrag zu den erblichen Nervenkrankheiten. Dtsch Z Nervenheilk 6:150–166
Hortobagyi T, Troakes C, Nishimura AL et al (2011) Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD–TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol 121:519–527
Hosler BA, Siddique T, Sapp PC et al (2000) Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21-q22. JAMA 284:1664–1669
Huang EJ, Zhang J, Geser F et al (2010) Extensive FUS-immunoreactive pathology in juvenile amyotrophic lateral sclerosis with basophilic inclusions. Brain Pathol 20:1069–1076
Huang C, Zhou H, Tong J et al (2011) FUS Transgenic Rats Develop the Phenotypes of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration. PLoS Genet 7:e1002011
Iida A, Takahashi A, Deng M et al (2011) Replication analysis of SNPs on 9p21.2 and 19p13.3 with amyotrophic lateral sclerosis in East Asians. Neurobiol Aging 32:757e713–757e754
Ito H, Fujita K, Nakamura M et al (2011) Optineurin is co-localized with FUS in basophilic inclusions of ALS with FUS mutation and in basophilic inclusion body disease. Acta Neuropathol 121:555–557
Johnson JO, Mandrioli J, Benatar M et al (2010) Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68:857–864
Ju JS, Weihl CC (2010) Inclusion body myopathy, Paget’s disease of the bone and fronto-temporal dementia: a disorder of autophagy. Hum Mol Genet 19:R38–R45
Ju JS, Fuentealba RA, Miller SE et al (2009) Valosin-containing protein (VCP) is required for autophagy and is disrupted in VCP disease. J Cell Biol 187:875–888
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574
Kawamata J, Shimohama S, Takano S, Harada K, Ueda K, Kimura J (1997) Novel G16S (GGC-AGC) mutation in the SOD-1 gene in a patient with apparently sporadic young-onset amyotrophic lateral sclerosis. Hum Mutat 9:356–358
Kovacs GG, Murrell JR, Horvath S et al (2009) TARDBP variation associated with frontotemporal dementia, supranuclear gaze palsy, and chorea. Mov Disord 24:1843–1847
Kwiatkowski TJ Jr, Bosco DA, Leclerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205–1208
Laaksovirta H, Peuralinna T, Schymick JC et al (2010) Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol 9:978–985
Lagier-Tourenne C, Polymenidou M, Cleveland DW (2010) TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19:R46–R64
Le Ber I, Camuzat A, Berger E et al (2009) Chromosome 9p-linked families with frontotemporal dementia associated with motor neuron disease. Neurology 72:1669–1676
Li Y, Ray P, Rao EJ et al (2010) A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci USA 107:3169–3174
Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60:1094–1097
Mackenzie IR, Baker M, West G et al (2006) A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain 129:853–867
Mackenzie IR, Bigio EH, Ince PG et al (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Mackenzie IR, Neumann M, Bigio EH et al (2010a) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Mackenzie IR, Rademakers R, Neumann M (2010b) TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol 9:995–1007
Maruyama H, Morino H, Ito H et al (2010) Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465:223–226
Matsuoka T, Fujii N, Kondo A et al (2011) An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology 31:71–76
Michou L, Collet C, Laplanche JL, Orcel P, Cornelis F (2006) Genetics of Paget’s disease of bone. Joint Bone Spine 73:243–248
Mitchell J, Paul P, Chen HJ et al (2010) Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci USA 107:7556–7561
Morita M, Al-Chalabi A, Andersen PM et al (2006) A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 66:839–844
Munch C, Rosenbohm A, Sperfeld AD et al (2005) Heterozygous R1101K mutation of the DCTN1 gene in a family with ALS and FTD. Ann Neurol 58:777–780
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishimura AL, Mitne-Neto M, Silva HC et al (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75:822–831
Orban P, Devon RS, Hayden MR, Leavitt BR (2007) Chapter 15 Juvenile amyotrophic lateral sclerosis. Handb Clin Neurol 82:301–312
Orlacchio A, Babalini C, Borreca A et al (2010) SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain 133:591–598
Osawa T, Mizuno Y, Fujita Y, Takatama M, Nakazato Y, Okamoto K (2011) Optineurin in neurodegenerative diseases. Neuropathology, in press
Parkinson N, Ince PG, Smith MO et al (2006) ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 67:1074–1077
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7:710–723
Pearson JP, Williams NM, Majounie E et al (2011) Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol 258:647–655
Puls I, Jonnakuty C, LaMonte BH et al (2003) Mutant dynactin in motor neuron disease. Nat Genet 33:455–456
Rezania K, Yan J, Dellefave L et al (2003) A rare Cu/Zn superoxide dismutase mutation causing familial amyotrophic lateral sclerosis with variable age of onset, incomplete penetrance and a sensory neuropathy. Amyotroph Lateral Scler Other Motor Neuron Disord 4:162–166
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE (2005) Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65:586–590
Rollinson S, Bennion J, Toulson G, et al. (2010) Analysis of optineurin in frontotemporal lobar degeneration. Neurobiol Aging, in press
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Shatunov A, Mok K, Newhouse S et al (2010) Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol 9:986–994
Siddique T, Pericak-Vance MA, Brooks BR et al (1989) Linkage analysis in familial amyotrophic lateral sclerosis. Neurology 39:919–925
Siddique T, Hong S, Brooks BR et al (1998) X-linked dominant ALS. Neurology 51:310–310
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Staal A, Went LN (1968) Juvenile amyotrophic lateral sclerosis-dementia complex in a Dutch family. Neurology 18:800–806
Suzuki N, Aoki M, Warita H et al (2010) FALS with FUS mutation in Japan, with early onset, rapid progress and basophilic inclusion. J Hum Genet 55:252–254
Talbot K, Ansorge O (2006) Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum Mol Genet 15(Spec No 2):R182–R187
Ticozzi N, Silani V, LeClerc AL et al (2009) Analysis of FUS gene mutation in familial amyotrophic lateral sclerosis within an Italian cohort. Neurology 73:1180–1185
Ticozzi N, LeClerc AL, Keagle PJ et al (2010) Paraoxonase gene mutations in amyotrophic lateral sclerosis. Ann Neurol 68:102–107
Ticozzi N, Tiloca C, Morelli C et al (2011) Genetics of familial amyotrophic lateral sclerosis. Arch Ital Biol 149:65–82
Valdmanis PN, Rouleau GA (2008) Genetics of familial amyotrophic lateral sclerosis. Neurology 70:144–152
Valdmanis PN, Dupre N, Bouchard JP et al (2007) Three families with amyotrophic lateral sclerosis and frontotemporal dementia with evidence of linkage to chromosome 9p. Arch Neurol 64:240–245
Valdmanis PN, Daoud H, Dion PA, Rouleau GA (2009) Recent advances in the genetics of amyotrophic lateral sclerosis. Curr Neurol Neurosci Rep 9:198–205
van Es MA, Diekstra FP, Veldink JH et al (2009a) A case of ALS–FTD in a large FALS pedigree with a K17I ANG mutation. Neurology 72:287–288
van Es MA, Veldink JH, Saris CG et al (2009b) Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet 41:1083–1087
Van Langenhove T, van der Zee J, Sleegers K et al (2010) Genetic contribution of FUS to frontotemporal lobar degeneration. Neurology 74:366–371
Vance C, Al-Chalabi A, Ruddy D et al (2006) Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 129:868–876
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Varelas PN, Bertorini TE, Kapaki E, Papageorgiou CT (1997) Paget’s disease of bone and motor neuron disease. Muscle Nerve 20:630
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 106:18809–18814
Weihl CC, Pestronk A, Kimonis VE (2009) Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord 19:308–315
Wightman G, Anderson VE, Martin J et al (1992) Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett 139:269–274
Wild P, Farhan H, McEwan DG et al (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333:228–233
Wils H, Kleinberger G, Janssens J et al (2010) TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 107:3858–3863
Yan J, Siddique N, Slifer S et al (2006) A major novel locus for ALS/FTD on chromosome 9p21 and its pathological correlates. Neurology 67:186-186-b
Yan JH, Slifer S, Siddique N et al (2007) Fine-mapping and candidate gene sequencing of the chromosome 9p locus of ALS/FTD. Neurology 68:A305-A305
Yan J, Deng HX, Siddique N et al (2010) Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia. Neurology 75:807–814
Yang Y, Hentati A, Deng HX et al (2001) The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet 29:160–165
Zarranz JJ, Ferrer I, Lezcano E et al (2005) A novel mutation (K317M) in the MAPT gene causes FTDP and motor neuron disease. Neurology 64:1578–1585
Zhou H, Huang C, Chen H et al (2010) Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet 6:e1000887
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fecto, F., Siddique, T. Making Connections: Pathology and Genetics Link Amyotrophic Lateral Sclerosis with Frontotemporal Lobe Dementia. J Mol Neurosci 45, 663–675 (2011). https://doi.org/10.1007/s12031-011-9637-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9637-9