Abstract
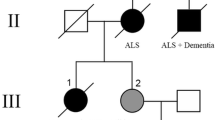
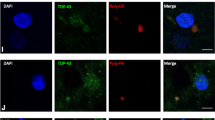
Optineurin (OPTN) is a multifunctional protein involved in vesicular trafficking, signal transduction and gene expression. OPTN mutations were described in eight Japanese patients with familial and sporadic amyotrophic lateral sclerosis (FALS, SALS). OPTN-positive inclusions co-localising with TDP-43 were described in SALS and in FALS with SOD-1 mutations, potentially linking two pathologically distinct pathways of motor neuron degeneration. We have explored the abundance of OPTN inclusions using a range of antibodies in postmortem tissues from 138 cases and controls including sporadic and familial ALS, frontotemporal lobar degeneration (FTLD) and a wide range of neurodegenerative proteinopathies. OPTN-positive inclusions were uncommon and detected in only 11/32 (34%) of TDP-43-positive SALS spinal cord and 5/15 (33%) of FTLD-TDP. Western blot of lysates from FTLD-TDP frontal cortex and TDP-43-positive SALS spinal cord revealed decreased levels of OPTN protein compared to controls (p < 0.05), however, this correlated with decreased neuronal numbers in the brain. Large OPTN inclusions were not detected in FALS with SOD-1 and FUS mutation, respectively, or in FTLD-FUS cases. OPTN-positive inclusions were identified in a few Alzheimer’s disease (AD) cases but did not co-localise with tau and TDP-43. Occasional striatal neurons contained granular cytoplasmic OPTN immunopositivity in Huntington’s disease (HD) but were absent in spinocerebellar ataxia type 3. No OPTN inclusions were detected in FTLD-tau and α-synucleinopathy. We conclude that OPTN inclusions are relatively rare and largely restricted to a minority of TDP-43 positive ALS and FTLD-TDP cases. Our results do not support the proposition that OPTN inclusions play a central role in the pathogenesis of ALS, FTLD or any other neurodegenerative disorder.




Similar content being viewed by others
References
Albagha OM, Visconti MR, Alonso N et al (2010) Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat Genet 42:520–524
Anborgh PH, Godin C, Pampillo M et al (2005) Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J Biol Chem 280:34840–34848
Brooks BR, Miller RG, Swash M et al (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cairns NJ, Bigio EH, Mackenzie IR et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22
Chung PY, Beyens G, Boonen S et al (2010) The majority of the genetic risk for Paget’s disease of bone is explained by genetic variants close to the CSF1, OPTN, TM7SF4, and TNFRSF11A genes. Hum Genet 128:615–626
De Marco N, Buono M, Troise F et al (2006) Optineurin increases cell survival and translocates to the nucleus in a Rab8-dependent manner upon an apoptotic stimulus. J Biol Chem 281:16147–16156
del Toro D, Alberch J, Lazaro-Dieguez F et al (2009) Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol Biol Cell 20:1478–1492
Greenway MJ, Andersen PM, Russ C et al (2006) ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet 38:411–413
Hattula K, Peranen J (2000) FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr Biol 10:1603–1606
Huber LA, Pimplikar S, Parton RG et al (1993) Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol 123:35–45
Hynd MR, Lewohl JM, Scott HL et al (2003) Biochemical and molecular studies using human autopsy brain tissue. J Neurochem 85:543–562
Kroeber M, Ohlmann A, Russell P et al (2006) Transgenic studies on the role of optineurin in the mouse eye. Exp Eye Res 82:1075–1085
Li Y, Kang J, Horwitz MS (1998) Interaction of an adenovirus E3 14.7-kilodalton protein with a novel tumor necrosis factor alpha-inducible cellular protein containing leucine zipper domains. Mol Cell Biol 18:1601–1610
Mackenzie IR, Neumann M, Bigio EH et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4
Maekawa S, Leigh PN, King A et al (2009) TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology 29:672–683
Maruyama H, Morino H, Ito H et al (2010) Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465:223–226
Moreau C, Devos D, Brunaud-Danel V et al (2005) Elevated IL-6 and TNF-alpha levels in patients with ALS: inflammation or hypoxia? Neurology 65:1958–1960
Moreland RJ, Dresser ME, Rodgers JS et al (2000) Identification of a transcription factor IIIA-interacting protein. Nucleic Acids Res 28:1986–1993
Nishimura AL, Mitne-Neto M, Silva HC et al (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75:822–831
Park B, Ying H, Shen X et al (2010) Impairment of protein trafficking upon overexpression and mutation of optineurin. PLoS One 5:e11547
Rezaie T, Child A, Hitchings R et al (2002) Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science 295:1077–1079
Rezaie T, Waitzman DM, Seeman JL et al (2005) Molecular cloning and expression profiling of optineurin in the rhesus monkey. Invest Ophthalmol Vis Sci 46:2404–2410
Rollinson S, Bennion J, Toulson G et al. Analysis of optineurin in frontotemporal lobar degeneration. Neurobiol Aging. doi:S0197-4580(0110)00425-00422
Rosen DR, Siddique T, Patterson D et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Sahlender DA, Roberts RC, Arden SD et al (2005) Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol 169:285–295
Shaw CE, Enayat ZE, Powell JF et al (1997) Familial amyotrophic lateral sclerosis. Molecular pathology of a patient with a SOD1 mutation. Neurology 49:1612–1616
Sreedharan J, Blair IP, Tripathi VB et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Sudhakar C, Nagabhushana A, Jain N et al (2009) NF-kappaB mediates tumor necrosis factor alpha-induced expression of optineurin, a negative regulator of NF-kappaB. PLoS One 4:e5114
Tezel G (2008) TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res 173:409–421
Vance C, Rogelj B, Hortobagyi T et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323:1208–1211
Velier J, Kim M, Schwarz C et al (1998) Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Exp Neurol 152:34–40
Ying H, Shen X, Park B et al (2010) Posttranslational modifications, localization, and protein interactions of optineurin, the product of a glaucoma gene. PLoS One 5:e9168
Zhu G, Wu CJ, Zhao Y et al (2007) Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr Biol 17:1438–1443
Acknowledgments
This work was supported in the UK by grants from Motor Neurone Disease Association UK; Medical Research Council UK; Wellcome Trust UK; The Psychiatry Research Trust of the Institute of Psychiatry; National Institute for Health Research Biomedical Research Centre for Mental Health at the South London and Maudsley National Health Service Foundation Trust and Institute of Psychiatry, King’s College London. The authors declare that they have no conflict of interest. The postmortem tissues were provided by the MRC London Neurodegenerative Diseases Brain Bank at the Institute of Psychiatry, King’s College London. We are thankful for the patients and their families who contributed to this research by giving consent for tissue donation for diagnosis and research. Technical assistance was provided by Christopher Bell, Vasiliki Spandoni, Olar Adeyi, Rita Monori and Marinela Vavla. We thank Emma Daniel and Hazel Urwin for helpful discussions and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Hortobágyi, C. Troakes and A. L. Nishimura contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hortobágyi, T., Troakes, C., Nishimura, A.L. et al. Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD-TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol 121, 519–527 (2011). https://doi.org/10.1007/s00401-011-0813-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0813-3