Abstract
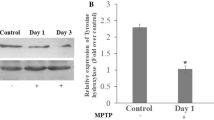
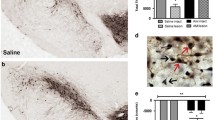
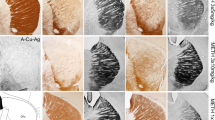
Our previous work had shown that long-term nicotine administration improved dopaminergic markers and nicotinic receptors (nAChRs) in the striatum of monkeys with nigrostriatal damage. The present experiments were done to determine whether nicotine treatment also led to changes in the substantia nigra, the region containing dopaminergic cell bodies. Monkeys were chronically treated with nicotine in the drinking water for 6 months after which they were injected with low dose of 1-methyl-4-phenyl-1,2,3,6-tetrahydrophridine (MPTP) for a further 6-month period. Nicotine was administered until the monkeys were euthanized 2 months after the last MPTP injection. Nicotine treatment did not affect the dopamine transporter or the number of tyrosine hydroxylase positive cells in the substantia nigra of lesioned monkeys. However, nicotine administration did lead to a greater increase in α3/α6β2* and α4β2* nAChRs in lesioned monkeys compared to controls. Nicotine also significantly elevated microglia and reduced the number of extracellular neuromelanin deposits in the substantia nigra of MPTP-lesioned monkeys. These findings indicate that long-term nicotine treatment modulates expression of several molecular measures in monkey substantia nigra that may result in an improvement in nigral integrity and/or function. These observations may have therapeutic implications for Parkinson’s disease.




Similar content being viewed by others
References
Allam, M. F., Campbell, M. J., Hofman, A., Del Castillo, A. S., & Fernandez-Crehuet Navajas, R. (2004). Smoking and Parkinson’s disease: Systematic review of prospective studies. Movement Disorders, 19, 614–621.
Bordia, T., Parameswaran, N., Fan, H., Langston, J. W., McIntosh, J. M., & Quik, M. (2006). Partial recovery of striatal nicotinic receptors in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-lesioned monkeys with chronic oral nicotine. Journal of Pharmacology and Experimental Therapeutics, 319, 285–292.
Bordia, T., Grady, S. R., McIntosh, J. M., & Quik, M. (2007). Nigrostriatal damage preferentially decreases a subpopulation of {alpha}6{beta}2* nAChRs in mouse, monkey and Parkinson's disease striatum. Molecular Pharmacology, 72, 52–61.
Carr, L. A., & Rowell, P. P. (1990). Attenuation of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neurotoxicity by tobacco smoke. Neuropharmacology, 29, 311–314.
Costa, G., Abin-Carriquiry, J. A., & Dajas, F. (2001). Nicotine prevents striatal dopamine loss produced by 6-hydroxydopamine lesion in the substantia nigra. Brain Research, 888, 336–342.
de Jonge, W. J., & Ulloa, L. (2007). The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. British Journal of Pharmacology, 151, 915–929.
Emmers, R., & Akert, K. (1963). A stereotaxic atlas of the brain of the squirrel monkey (Saimiri sciureus). Madison: University of Wisconsin Press.
Fasano, M., Bergamasco, B., & Lopiano, L. (2006). Modifications of the iron-neuromelanin system in Parkinson's disease. Journal of Neurochemistry, 96, 909–916.
Gibb, W. R. (1992). Melanin, tyrosine hydroxylase, calbindin and substance P in the human midbrain and substantia nigra in relation to nigrostriatal projections and differential neuronal susceptibility in Parkinson's disease. Brain Research, 581, 283–291.
Gotti, C., Moretti, M., Clementi, F., Riganti, L., McIntosh, J. M., Collins, A. C., et al. (2005). Expression of nigrostriatal alpha 6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by beta 3 subunit gene deletion. Molecular Pharmacology, 67, 2007–2015.
Gwilt, C. R., Donnelly, L. E., & Rogers, D. F. (2007). The non-neuronal cholinergic system in the airways: An unappreciated regulatory role in pulmonary inflammation? Pharmacology and Therapeutics, 115, 208–222.
Janson, A. M., Fuxe, K., Agnati, L. F., Kitayama, I., Harfstrand, A., Andersson, K., et al. (1988). Chronic nicotine treatment counteracts the disappearance of tyrosine-hydroxylase-immunoreactive nerve cell bodies, dendrites and terminals in the mesostriatal dopamine system of the male rat after partial hemitransection. Brain Research, 455, 332–345.
Kastner, A., Hirsch, E. C., Lejeune, O., Javoy-Agid, F., Rascol, O., & Agid, Y. (1992). Is the vulnerability of neurons in the substantia nigra of patients with Parkinson's disease related to their neuromelanin content? Journal of Neurochemistry, 59, 1080–1089.
Keath, J. R., Iacoviello, M. P., Barrett, L. E., Mansvelder, H. D., & McGehee, D. S. (2007). Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. Journal of Neurophysiology, 98, 3388–3396.
Khwaja, M., McCormack, A., McIntosh, J. M., Di Monte, D. A., & Quik, M. (2007). Nicotine partially protects against paraquat-induced nigrostriatal damage in mice; link to alpha6beta2* nAChRs. Journal of Neurochemistry, 100, 180–190.
Kulak, J. M., McIntosh, J. M., & Quik, M. (2002). Loss of nicotinic receptors in monkey striatum after 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine treatment is due to a decline in alpha-conotoxin MII sites. Molecular Pharmacology, 61, 230–238.
Kulak, J. M., Musachio, J. L., McIntosh, J. M., & Quik, M. (2002). Declines in different beta2* nicotinic receptor populations in monkey striatum after nigrostriatal damage. Journal of Pharmacology and Experimental Therapeutics, 303, 633–639.
Lai, A., Parameswaran, N., Khwaja, M., Whiteaker, P., Lindstrom, J. M., Fan, H., et al. (2005). Long-term nicotine treatment decreases striatal alpha6* nicotinic acetylcholine receptor sites and function in mice. Molecular Pharmacology, 67, 1639–1647.
Marks, M. J., Pauly, J. R., Gross, S. D., Deneris, E. S., Hermans-Borgmeyer, I., Heinemann, S. F., et al. (1992). Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. Journal of Neuroscience, 12, 2765–2784.
Matta, S. G., Balfour, D. J., Benowitz, N. L., Boyd, R. T., Buccafusco, J. J., Caggiula, A. R., et al. (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl), 190, 269–319.
McCallum, S. E., Parameswaran, N., Bordia, T., Fan, H., Tyndale, R. F., Langston, J. W., et al. (2006). Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. Journal of Neurochemistry, 96, 1028–1041.
McGeer, P. L., Itagaki, S., Boyes, B. E., & McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology, 38, 1285–1291.
McIntosh, J. M., Azam, L., Staheli, S., Dowell, C., Lindstrom, J. M., Kuryatov, A., et al. (2004). Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Molecular Pharmacology, 65, 944–952.
Morens, D. M., Grandinetti, A., Reed, D., White, L. R., & Ross, G. W. (1995). Cigarette smoking and protection from Parkinson’s disease: False association or etiologic clue? Neurology, 45, 1041–1051.
Mugnaini, M., Garzotti, M., Sartori, I., Pilla, M., Repeto, P., Heidbreder, C. A., et al. (2006). Selective down-regulation of [(125)I]Y(0)-alpha-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience, 137, 565–572.
Nagatsu, T., & Sawada, M. (2006). Cellular and molecular mechanisms of Parkinson’s disease: Neurotoxins, causative genes, and inflammatory cytokines. Cellular and Molecular Neurobiology, 26, 781–802.
Nashmi, R., Xiao, C., Deshpande, P., McKinney, S., Grady, S. R., Whiteaker, P., et al. (2007). Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. Journal of Neuroscience, 27, 8202–8218.
Nguyen, H. N., Rasmussen, B. A., & Perry, D. C. (2003). Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. Journal of Pharmacology and Experimental Therapeutics, 307, 1090–1097.
Park, H. J., Lee, P. H., Ahn, Y. W., Choi, Y. J., Lee, G., Lee, D. Y., et al. (2007). Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. European Journal of Neuroscience, 26, 79–89.
Perry, D. C., Mao, D., Gold, A. B., McIntosh, J. M., Pezzullo, J. C., & Kellar, K. J. (2007). Chronic nicotine differentially regulates {alpha}6- and {beta}3-containing nicotinic cholinergic receptors in rat brain. Journal of Pharmacology and Experimental Therapeutics, 322, 306–315.
Picciotto, M. R., & Zoli, M. (2008). Neuroprotection via nAChRs: The role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Frontiers in Bioscience, 13, 492–504.
Pons, S., Fattore, L., Cossu, G., Tolu, S., Porcu, E., McIntosh, J. M., et al. (2008). Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. Journal of Neuroscience, 28, 12318–12327.
Quik, M., Polonskaya, Y., Gillespie, A., Lloyd, G. K., & Langston, J. W. (2000). Differential alterations in nicotinic receptor alpha6 and beta3 subunit messenger RNAs in monkey substantia nigra after nigrostriatal degeneration. Neuroscience, 100, 63–72.
Quik, M., Polonskaya, Y., Kulak, J. M., & McIntosh, J. M. (2001). Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. Journal of Neuroscience, 21, 5494–5500.
Quik, M., Polonskaya, Y., McIntosh, J. M., & Kulak, J. M. (2002). Differential nicotinic receptor expression in monkey basal ganglia: Effects of nigrostriatal damage. Neuroscience, 112, 619–630.
Quik, M., Vailati, S., Bordia, T., Kulak, J. M., Fan, H., McIntosh, J. M., et al. (2005). Subunit composition of nicotinic receptors in monkey striatum: Effect of treatments with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine or L-DOPA. Molecular Pharmacology, 67, 32–41.
Quik, M., Chen, L., Parameswaran, N., Xie, X., Langston, J. W., & McCallum, S. E. (2006). Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned primates. Journal of Neuroscience, 26, 4681–4689.
Quik, M., Parameswaran, N., McCallum, S. E., Bordia, T., Bao, S., McCormack, A., et al. (2006). Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. Journal of Neurochemistry, 98, 1866–1875.
Quik, M., O'Neill, M., & Perez, X. A. (2007). Nicotine neuroprotection against nigrostriatal damage: Importance of the animal model. Trends in Pharmacological Sciences, 28(5), 229–235.
Rao, K. S., Hegde, M. L., Anitha, S., Musicco, M., Zucca, F. A., Turro, N. J., et al. (2006). Amyloid beta and neuromelanin–toxic or protective molecules? The cellular context makes the difference. Progress in Neurobiology, 78, 364–373.
Ritz, B., Ascherio, A., Checkoway, H., Marder, K. S., Nelson, L. M., Rocca, W. A., et al. (2007). Pooled analysis of tobacco use and risk of Parkinson disease. Archives of Neurology, 64, 990–997.
Ryan, R. E., Ross, S. A., Drago, J., & Loiacono, R. E. (2001). Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. British Journal of Pharmacology, 132, 1650–1656.
Sawada, M., Imamura, K., & Nagatsu, T. (2006). Role of cytokines in inflammatory process in Parkinson's disease. Journal of Neural Transmission. Supplementum, 373–381.
Shytle, R. D., Mori, T., Townsend, K., Vendrame, M., Sun, N., Zeng, J., et al. (2004). Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. Journal of Neurochemistry, 89, 337–343.
Visanji, N. P., O'Neill, M. J., & Duty, S. (2006). Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology, 51, 506–516.
Wersinger, C., & Sidhu, A. (2006). An inflammatory pathomechanism for Parkinson's disease? Current Medicinal Chemistry, 13, 591–602.
Whiteaker, P., McIntosh, J. M., Luo, S., Collins, A. C., & Marks, M. J. (2000). 125I-alpha-conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Molecular Pharmacology, 57, 913–925.
Zecca, L., Fariello, R., Riederer, P., Sulzer, D., Gatti, A., & Tampellini, D. (2002). The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Letters, 510, 216–220.
Zoli, M., Moretti, M., Zanardi, A., McIntosh, J. M., Clementi, F., & Gotti, C. (2002). Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. Journal of Neuroscience, 22, 8785–8789.
Acknowledgements
The authors thank Dr. Hong Fan, Department of Radiology, Johns Hopkins University School of Medicine, for the 125I-A85380 and 125I-epibatidine used in this study. This work is supported by NIH grants NS42091 and U54 ES012077 to MQ, and MH53631, GM 48677 and DA12242 to JMM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings of the XIII International Symposium on Cholinergic Mechanisms
Rights and permissions
About this article
Cite this article
Quik, M., Campos, C., Parameswaran, N. et al. Chronic Nicotine Treatment Increases nAChRs and Microglial Expression in Monkey Substantia Nigra After Nigrostriatal Damage. J Mol Neurosci 40, 105–113 (2010). https://doi.org/10.1007/s12031-009-9265-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9265-9