Abstract
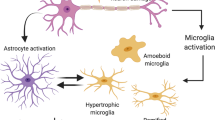
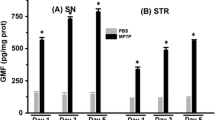
The precise mechanism(s) of Parkinson’s disease, a progressive neurodegenerative disorder affecting a large number of people worldwide, is far from clearly understood. For disease induction in mouse model, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is used that specifically destroys those neurons of basal ganglia and the substantia nigra that are involved in Parkinsonism. However, the effect of MPTP treatment on glial cells that play significant role in maintaining brain homeostasis remains unexplored. In view of this, the present study was designed to investigate the status of microglia and astrocytes in substantia nigra as well as in hippocampus, the region related to memory processing and cognition, in mouse brain upon MPTP administration. We examined the neuropathological alterations in hippocampus and substantia nigra upon MPTP administration by using western blot, quantitatitative PCR, immunohistochemistry and immunofluorescence. Subcutaneous administration of MPTP in Swiss mice resulted in degeneration of nigrostriatal dopaminergic neurons. In addition, there was marked microglial activation and neuroinflammation in both the substantia nigra and the hippocampus. Astrocytes also showed activation at early phase (day 1 post treatment), but it was substantially reduced in substantia nigra at day 3 post treatment. However, the number of astrocytes remained reduced in the hippocampus throughout the post-treatment period. Consistent microglial activation and neuroinflammation with gradual decline in the number of astrocytes in both substantia nigra and hippocampus appear causally associated with MPTP-induced progressive degeneration of mouse brain.





Similar content being viewed by others
References
Furuya, T., H. Hayakawa, M. Hayakawa, K. Yoshimi, S. Hisahara, M. Miura, Y. Mizuno, and H. Mochizuki. 2004. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. J Neurosci 8: 1865–1872.
Ghosh, N., S. Mitra, P. Sinha, N. Chakrabarti, and A. Bhattacharyya. 2018. TNFR2 mediated TNF-α signaling and NF-κB activation in hippocampus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. Neurosci Res 137: 36–42.
Glass, C.K., K. Saijo, B. Winner, M.C. Marchetto, and F.H. Gage. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 6: 918–934.
Huang, D., J. Xu, J. Wang, J. Tong, X. Bai, H. Li, Z. Wang, Y. Huang, Y. Wu, M. Yu, and F. Huang. 2017. Dynamic changes in the nigrostriatal pathway in the MPTP mouse model of Parkinson’s disease. Parkinsons Dis 2017: 9349487.
Jackson-Lewis, V., and S. Przedborski. 2007. Protocol for the MPTP mouse model of Parkinson’s disease. Nature Protocols 1: 141–151.
Kempermann, G., H. Song, and F.H. Gage. 2015. Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 9: a018812.
Kettenmann, H., U.K. Hanisch, M. Noda, and A. Verkhratsky. 2011. Physiology of microglia. Physiol Rev 2: 461–553.
Kimelberg, H.K., and M. Nedergaard. 2010. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 4: 338–353.
Knierim, J.J. 2015. The hippocampus. Curr Biol 23: R1116–R1121.
Langston, J.W., L.S. Forno, J. Tetrud, A.G. Reeves, J.A. Kaplan, and D. Karluk. 1999. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 4: 598–605.
Lawson, L.J., V.H. Perry, P. Dri, and S. Gordon. 1990. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 1: 151–170.
Lee, S., J.Y. Park, W.H. Lee, H. Kim, H.C. Park, K. Mori, and K. Suk. 2009. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci 1: 234–249.
L’Episcopo, F., C. Tirolo, N. Testa, S. Caniglia, M.C. Morale, M. Deleidi, M.F. Serapide, S. Pluchino, and B. Marchetti. 2012. Plasticity of subventricular zone neuroprogenitors in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease involves cross talk between inflammatory and Wnt/β-catenin signaling pathways: functional consequences for neuroprotection and repair. J Neurosci 6: 2062–2085.
Liu, J., D. Huang, J. Xu, J. Tong, Z. Wang, L. Huang, Y. Yang, X. Bai, P. Wang, H. Suo, Y. Ma, M. Yu, J. Yu, and F. Huang. 2015. Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci Rep 5: 15720.
McGeer, P.L., and E.G. McGeer. 2008. Glial reactions in Parkinson’s disease. Mov Disord 4: 474–483.
McGeer, P.L., S. Itagaki, B.E. Boyes, and E.G. McGeer. 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 8: 1285–1291.
Mitra, S., N. Ghosh, P. Sinha, N. Chakrabarti, and A. Bhattacharyya. 2015. Alteration in nuclear factor-kappaB pathway and functionality of estrogen via receptors promote neuroinflammation in frontal cortex after 1-Methyl-4-Phenyl-1,2,3,6-tetrahydropyridine treatment. Sci Rep 5: 13949.
Rodrigues, M.C., P.R. Sanberg, L.E. Cruz, and S. Garbuzova-Davis. 2014. The innate and adaptive immunological aspects in neurodegenerative diseases. J Neuroimmunol 1–2: 1–8.
Saijo, K., and C.K. Glass. 2011. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787.
Saijo, K., B. Winner, C.T. Carson, J.G. Collier, L. Boyer, M.G. Rosenfeld, F.H. Gage, and C.K. Glass. 2009. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 1: 47–59.
Suzumura, A. 2013. Neuron-microglia interaction in neuroinflammation. Curr Protein Pept Sci 1: 16–20.
Sy, H.N., S.L. Wu, W.F. Wang, C.H. Chen, Y.T. Huang, Y.M. Liou, C.S. Chiou, C.R. Pawlak, and Y.J. Ho. 2010. MPTP-induced dopaminergic degeneration and deficits in object recognition in rats are accompanied by neuroinflammation in the hippocampus. Pharmacol Biochem Behav 2: 158–165.
Wang, Q., Y. Liu, and J. Zhou. 2015. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4: 19.
Watson, G.S., and J.B. Leverenz. 2010. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol 3: 640–645.
Yamada, T., T. Kawamata, D.G. Walker, and P.L. McGeer. 1992. Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol 2: 157–162.
Yan, Y., W. Jiang, L. Liu, X. Wang, C. Ding, Z. Tian, and R. Zhou. 2015. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 1–2: 62–73.
Yu, M., H. Suo, M. Liu, L. Cai, J. Liu, Y. Huang, J. Xu, Y. Wang, C. Zhu, J. Fei, and F. Huang. 2013. Neurobiol Aging 3: 916–927.
Acknowledgements
The authors are grateful to Neuroscience Taskforce, Department of Biotechnology (Govt. of India) for funding this work and providing fellowship support (Project Sanction No. BT/PR3986/MED/30/677/2011). Authors are grateful to the Animal Ethical Committee for providing the ethical clearance to execute the animal experiments. Authors are thankful to Maulana Azad College, Dept. of Zoology, for assistance in Gel Documentation and Light microscopy. Authors express their sincere thanks to all lab members and friends for lending their helping hands in animal maintenance and other assistance whenever necessary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, N., Mitra, S., Sinha, P. et al. Study of Microglial and Astroglial Alterations Induced by Acute 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Treatment in Mouse Brain. Proc Zool Soc 73, 32–39 (2020). https://doi.org/10.1007/s12595-019-00296-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-019-00296-4