Abstract
In this review, we detail the changes and the relevant features that are applied to neuroendocrine neoplasms (NENs) in the 2022 WHO Classification of Endocrine and Neuroendocrine Tumors. Using a question-and-answer approach, we discuss the consolidation of the nomenclature that distinguishes neuronal paragangliomas from epithelial neoplasms, which are divided into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). The criteria for these distinctions based on differentiation are outlined. NETs are generally (but not always) graded as G1, G2, and G3 based on proliferation, whereas NECs are by definition high grade; the importance of Ki67 as a tool for classification and grading is emphasized. The clinical relevance of proper classification is explained, and the importance of hormonal function is examined, including eutopic and ectopic hormone production. The tools available to pathologists for accurate classification include the conventional biomarkers of neuroendocrine lineage and differentiation, INSM1, synaptophysin, chromogranins, and somatostatin receptors (SSTRs), but also include transcription factors that can identify the site of origin of a metastatic lesion of unknown primary site, as well as hormones, enzymes, and keratins that play a role in functional and structural correlation. The recognition of highly proliferative, well-differentiated NETs has resulted in the need for biomarkers that can distinguish these G3 NETs from NECs, including stains to determine expression of SSTRs and those that can indicate the unique molecular pathogenetic alterations that underlie the distinction, for example, global loss of RB and aberrant p53 in pancreatic NECs compared with loss of ATRX, DAXX, and menin in pancreatic NETs. Other differential diagnoses are discussed with recommendations for biomarkers that can assist in correct classification, including the distinctions between epithelial and non-epithelial NENs that have allowed reclassification of epithelial NETs in the spine, in the duodenum, and in the middle ear; the first two may be composite tumors with neuronal and glial elements, and as this feature is integral to the duodenal lesion, it is now classified as composite gangliocytoma/neuroma and neuroendocrine tumor (CoGNET). The many other aspects of differential diagnosis are detailed with recommendations for biomarkers that can distinguish NENs from non-neuroendocrine lesions that can mimic their morphology. The concepts of mixed neuroendocrine and non-neuroendocrine (MiNEN) and amphicrine tumors are clarified with information about how to approach such lesions in routine practice. Theranostic biomarkers that assist patient management are reviewed. Given the significant proportion of NENs that are associated with germline mutations that predispose to this disease, we explain the role of the pathologist in identifying precursor lesions and applying molecular immunohistochemistry to guide genetic testing.







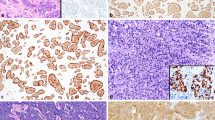
reproduced from Hackeng et al. [65]












Similar content being viewed by others
Availability of Data and Material
Not applicable.
References
Bayliss WM, Starling EH (1902) The mechanism of pancreatic secretion. J Physiol 28: 325-353.
Cushing H (1909) III. Partial Hypophysectomy for Acromegaly: With Remarks on the Function of the Hypophysis. Ann Surg 50: 1002-1017. https://doi.org/10.1097/00000658-190912000-00003 [doi].
Minkowski O (1887) Ueber einen Fall von Akromegalie. Berl Klin Wochenschr 24: 371-374.
Oberndorfer S (1907) Karzinoide tumoren des Dünndarms. Frankfurter Zeitschrift für Pathologie 1: 425-432.
Thorson A, Biorck G, Bjorkman G, Waldenstrom J (1954) Malignant carcinoid of the small intestine with metastases to the liver, valvular disease of the right side of the heart (pulmonary stenosis and tricuspid regurgitation without septal defects), peripheral vasomotor symptoms, bronchoconstriction, and an unusual type of cyanosis; a clinical and pathologic syndrome. Am Heart J 47: 795–817. 0002–8703(54)90152–0 ; https://doi.org/10.1016/0002-8703(54)90152-0.
Ransom W (1890) A case of primary carcinoma of the ileum. Lancet 136: 1020-1023.
Asa SL, Lloyd RV, Tischler AS (2021) Neuroendocrine neoplasms: Historical background and terminologies. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham, Switzerland: Springer Nature. pp. 1–14.
Travis, W. D., Burke, A. P., Marx, A., and Nicholson, A. G. (2015) WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC.
Bosman FT, Carneiro F, Hruban RH, Teiise ND (2010) WHO Classification of Tumours of the Gastrointestinal Tract.
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Kloppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA (2018) A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31: 1770-1786. https://doi.org/10.1038/s41379-018-0110-y.
Rindi G, Wiedenmann B (2011) Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol 8: 54-64. https://doi.org/10.1038/nrendo.2011.120.
Rosenbaum JN, Guo Z, Baus RM, Werner H, Rehrauer WM, Lloyd RV (2015) INSM1: A Novel Immunohistochemical and Molecular Marker for Neuroendocrine and Neuroepithelial Neoplasms. Am J Clin Pathol 144: 579–591. 144/4/579; https://doi.org/10.1309/AJCPGZWXXBSNL4VD.
Mete O, Asa SL, Gill AJ, Kimuta N, deKrijger R, Tischler A (2022) Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr Pathol.
Klimstra D, Kloppel G, La Rosa S, Rindi G (2019) Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification od Tumours Editorial Board, editors. Digestive System Tumours. Lyon: IARC Press. pp. 16-19.
Travis WD, Beasley M, Cree I, Papotti M, Rekhtman N, et al. (2022) Lung Neuroendocrine Neoplasms. In: WHO Classification of Tumours Editorial Board, editors. Thoracic Tumours. Lyon: IARC Press. pp. 109-111.
WHO Classification of Tumours Editorial Board (2022) WHO classification of endocrine and neuroendocrine tumours. Lyon, France: IARC.
Drucker DJ, Asa SL, Henderson J, Goltzman D (1989) The parathyroid hormone-like peptide gene is expressed in the normal and neoplastic human endocrine pancreas. Mol Endocrinol 3: 1589-1595.
Asa SL, Henderson J, Goltzman D, Drucker DJ (1990) Parathyroid hormone-like peptide in normal and neoplastic human endocrine tissues. J Clin Endocrinol Metab 71: 1112-1118.
Ezzat S, Ezrin C, Yamashita S, Melmed S (1993) Recurrent acromegaly resulting from ectopic growth hormone gene expression by a metastatic pancreatic tumor. Cancer 71: 66-70.
Alshaikh OM, Yoon JY, Chan BA, Krzyzanowska MK, Butany J, Asa SL, Ezzat S (2018) Pancreatic Neuroendocrine Tumor Producing Insulin and Vasopressin. Endocr Pathol 29: 15-20. https://doi.org/10.1007/s12022-017-9492-5.
Asa SL, Kovacs K, Vale W, Petrusz P, Vecsei P (1987) Immunohistologic localization of corticotrophin-releasing hormone in human tumors. Am J Clin Pathol 87: 327-333.
Maragliano R, Vanoli A, Albarello L, Milione M, Basturk O, Klimstra DS, Wachtel A, Uccella S, Vicari E, Milesi M, Davi MV, Scarpa A, Sessa F, Capella C, La Rosa S (2015) ACTH-secreting pancreatic neoplasms associated with Cushing syndrome: clinicopathologic study of 11 cases and review of the literature. Am J Surg Pathol 39: 374-382. https://doi.org/10.1097/PAS.0000000000000340.
Fountas A, Giotaki Z, Ligkros N, Tsakiridou ED, Tigas S, Saeger W, Tsatsoulis A (2015) Cushing’s Syndrome Due to CRH and ACTH Co-secreting Pancreatic Tumor--Presentation of a New Case Focusing on Diagnostic Pitfalls. Endocr Pathol 26: 239-242. https://doi.org/10.1007/s12022-015-9384-5.
Suissa Y, Magenheim J, Stolovich-Rain M, Hija A, Collombat P, Mansouri A, Sussel L, Sosa-Pineda B, McCracken K, Wells JM, Heller RS, Dor Y, Glaser B (2013) Gastrin: a distinct fate of neurogenin3 positive progenitor cells in the embryonic pancreas. PLoS ONE 8: e70397. https://doi.org/10.1371/journal.pone.0070397.
Kamp K, Feelders RA, van Adrichem RC, de Rijke YB, van Nederveen FH, Kwekkeboom DJ, de Herder WW (2014) Parathyroid hormone-related peptide (PTHrP) secretion by gastroenteropancreatic neuroendocrine tumors (GEP-NETs): clinical features, diagnosis, management, and follow-up. J Clin Endocrinol Metab 99: 3060-3069. https://doi.org/10.1210/jc.2014-1315.
Sano T, Asa SL, Kovacs K (1988) Growth hormone-releasing hormone-producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev 9: 357-373.
Sano T, Saito H, Yamasaki R, Kimura N, Sasaki A, Hirose T, Hizawa K, Saito S (1987) Immunoreactivity against anti-growth hormone-releasing hormone (GHRH) sera in human pancreas and pancreatic endocrine tumors: Evidence of pitfall in immunohistochemical study. Biomed Res 8: 407-414.
Uccella S, Blank A, Maragliano R, Sessa F, Perren A, La Rosa S (2017) Calcitonin-Producing Neuroendocrine Neoplasms of the Pancreas: Clinicopathological Study of 25 Cases and Review of the Literature. Endocr Pathol 28: 351-361. https://doi.org/10.1007/s12022-017-9505-4.
Samyn I, Fontaine C, Van TF, Pipeleers-Marichal M, De GJ (2004) Paraneoplastic syndromes in cancer: Case 1. Polycythemia as a result of ectopic erythropoietin production in metastatic pancreatic carcinoid tumor. J Clin Oncol 22: 2240-2242. https://doi.org/10.1200/JCO.2004.10.031.
Miras AD, Mogford JT, Wright J, Mendoza NN, Xekouki P, Lakhani A, Pellegata NS, Stratakis CA, Roncaroli F, Russell-Jones D (2015) Ovarian hyperstimulation from ectopic hypersecretion of follicle stimulating hormone. Lancet 385: 392. https://doi.org/10.1016/S0140-6736(14)62294-7.
Piaditis G, Angellou A, Kontogeorgos G, Mazarakis N, Kounadi T, Kaltsas G, Vamvakidis K, Lloyd RV, Horvath E, Kovacs K (2005) Ectopic bioactive luteinizing hormone secretion by a pancreatic endocrine tumor, manifested as luteinized granulosa-thecal cell tumor of the ovaries. J Clin Endocrinol Metab 90: 2097-2103. https://doi.org/10.1210/jc.2003-032029.
Brignardello E, Manti R, Papotti M, Allia E, Campra D, Isolato G, Cassinis MC, Fronda G, Boccuzzi G (2004) Ectopic secretion of LH by an endocrine pancreatic tumor. J Endocrinol Invest 27: 361-365.https://doi.org/10.1007/BF03351063 .
Mete O, Hannah-Shmouni F, Kim R, Stratakis C (2021) Inherited neuroendocrine neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham, Switzerland: Springer Nature. pp. 409–460.
Okubo Y, Yoshioka E, Suzuki M, Washimi K, Kawachi K, Kameda Y, Yokose T (2018) Diagnosis, Pathological Findings, and Clinical Management of Gangliocytic Paraganglioma: A Systematic Review. Front Oncol 8: 291. https://doi.org/10.3389/fonc.2018.00291.
Ramani B, Gupta R, Wu J, Barreto J, Bollen AW, Tihan T, Mummaneni PV, Ames C, Clark A, Oberheim Bush NA, Butowski N, Phillips D, King BE, Bator SM, Treynor EC, Zherebitskiy V, Quinn PS, Walker JB, Pekmezci M, Sullivan DV, Hofmann JW, Sloan EA, Chang M, Berger MS, Solomon DA, Perry A (2020) The immunohistochemical, DNA methylation, and chromosomal copy number profile of cauda equina paraganglioma is distinct from extra-spinal paraganglioma. Acta Neuropathol 140: 907-917. https://doi.org/10.1007/s00401-020-02221-y.
Hofland J, Kaltsas G, de Herder WW (2020) Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr Rev 41: 5573926. https://doi.org/10.1210/endrev/bnz004.
Chbat J, Amer L, Akirov A, Ezzat S (2021) The diagnosis of neuroendocrine neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham, Switzerland: Springer Nature. pp. 15–28.
Asa SL , Ezzat S, Mete O (2018) The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations J Clin Med 7.https://doi.org/10.3390/jcm7090280
Akirov A, Asa SL, Amer L, Shimon I, Ezzat S (2019) The Clinicopathological Spectrum of Acromegaly J Clin Med 8.https://doi.org/10.3390/jcm8111962
Dromain C, Prior JO, Schaefer N (2021) Functional and radiological imaging of neuroendocrine neoplasms. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Cham, Switzerland: Spinder Nature. pp. 29–54.
Akirov A, Larouche V, Alshehri S, Asa SL, Ezzat S (2019) Treatment Options for Pancreatic Neuroendocrine Tumors Cancers (Basel) 11.https://doi.org/10.3390/cancers11060828
Buscombe JR (2020) Evidence Base for the Use of PRRT. Semin Nucl Med 50: 399-404. https://doi.org/10.1053/j.semnuclmed.2020.04.001.
Haug AR (2020) PRRT of neuroendocrine tumors: individualized dosimetry or fixed dose scheme? EJNMMI Res 10: 35. https://doi.org/10.1186/s13550-020-00623-3.
Shaheen S, Moradi F, Gamino G, Kunz PL (2020) Patient Selection and Toxicities of PRRT for Metastatic Neuroendocrine Tumors and Research Opportunities. Curr Treat Options Oncol 21: 25. https://doi.org/10.1007/s11864-020-0711-9.
Tsai HK, Hornick JL, Vivero M (2020) INSM1 expression in a subset of thoracic malignancies and small round cell tumors: rare potential pitfalls for small cell carcinoma. Mod Pathol 33: 1571-1580. https://doi.org/10.1038/s41379-020-0517-0.
Kanitra JJ, Hardaway JC, Soleimani T, Koehler TJ, McLeod MK, Kavuturu S (2018) Adrenocortical oncocytic neoplasm: A systematic review. Surgery 164: 1351-1359. https://doi.org/10.1016/j.surg.2018.04.044.
Ohara Y, Oda T, Hashimoto S, Akashi Y, Miyamoto R, Enomoto T, Satomi K, Morishita Y, Ohkohchi N (2016) Pancreatic neuroendocrine tumor and solid-pseudopapillary neoplasm: Key immunohistochemical profiles for differential diagnosis. World J Gastroenterol 22: 8596-8604. https://doi.org/10.3748/wjg.v22.i38.8596.
Rindi G, Wiedenmann B (2020) Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine. Nat Rev Endocrinol 16: 590-607. https://doi.org/10.1038/s41574-020-0391-3.
Asa, S. L. (2020) Survival Guide to Endocrine Pathology. Virginia: Innovative Pathology Press.
Duan K, Mete O (2016) Algorithmic approach to neuroendocrine tumors in targeted biopsies: Practical applications of immunohistochemical markers. Cancer Cytopathol 124: 871-884. https://doi.org/10.1002/cncy.21765.
Uccella S, Asa SL, Mete O (2020) Neuroendocrine neoplasms of unknown primary site. In: Asa SL, La Rosa S, Mete O, editors. The Spectrum of Neuroendocrine Neoplasia. Springer.
Uccella S, La Rosa S, Volante M, Papotti M (2018) Immunohistochemical Biomarkers of Gastrointestinal, Pancreatic, Pulmonary, and Thymic Neuroendocrine Neoplasms. Endocr Pathol 29: 150-168. https://doi.org/10.1007/s12022-018-9522-y.
Mete O, Kefeli M, Caliskan S, Asa SL (2019) GATA3 immunoreactivity expands the transcription factor profile of pituitary neuroendocrine tumors. Mod Pathol 32: 484-489. https://doi.org/10.1038/s41379-018-0167-7.
Asa SL, Mete O, Cusimano MD, McCutcheon IE, Perry A, Yamada S, Nishioka H, Casar-Borota O, Uccella S, La Rosa S, Grossman AB, Ezzat S (2021) Pituitary neuroendocrine tumors: a model for neuroendocrine tumor classification. Mod Pathol 34: 1634-1650. https://doi.org/10.1038/s41379-021-00820-y.
Baloch Z, Mete O, Asa SL (2018) Immunohistochemical Biomarkers in Thyroid Pathology. Endocr Pathol 29: 91-112. https://doi.org/10.1007/s12022-018-9532-9.
Gucer H, Caliskan S, Kefeli M, Mete O (2020) Do You Know the Details of Your PAX8 Antibody? Monoclonal PAX8 (MRQ-50) Is Not Expressed in a Series of 45 Medullary Thyroid Carcinomas. Endocr Pathol 31: 33-38. https://doi.org/10.1007/s12022-019-09603-3.
Liau JY, Tsai JH, Jeng YM, Kuo KT, Huang HY, Liang CW, Yang CY (2016) The Diagnostic Utility of PAX8 for Neuroendocrine Tumors: An Immunohistochemical Reappraisal. Appl Immunohistochem Mol Morphol 24: 57-63. https://doi.org/10.1097/PAI.0000000000000149.
Erickson LA, Mete O (2018) Immunohistochemistry in Diagnostic Parathyroid Pathology. Endocr Pathol 29: 113-129. https://doi.org/10.1007/s12022-018-9527-6.
Hoskoppal D, Epstein JI, Gown AM, Arnold Egloff SA, Gordetsky JB, Shi CJ, Giannico GA (2020) SATB2 protein expression by immunohistochemistry is a sensitive and specific marker of appendiceal and rectosigmoid well differentiated neuroendocrine tumours. Histopathology 76: 550-559. https://doi.org/10.1111/his.14012.
Hackeng WM, Brosens LAA, Kim JY, O'Sullivan R, Sung YN, Liu TC, Cao D, Heayn M, Brosnan-Cashman J, An S, Morsink FHM, Heidsma CM, Valk GD, Vriens MR, Nieveen van DE, Offerhaus GJA, Dreijerink KMA, Zeh H, Zureikat AH, Hogg M, Lee K, Geller D, Marsh JW, Paniccia A, Ongchin M, Pingpank JF, Bahary N, Aijazi M, Brand R, Chennat J, Das R, Fasanella KE, Khalid A, McGrath K, Sarkaria S, Singh H, Slivka A, Nalesnik M, Han X, Nikiforova MN, Lawlor RT, Mafficini A, Rusev B, Corbo V, Luchini C, Bersani S, Pea A, Cingarlini S, Landoni L, Salvia R, Milione M, Milella M, Scarpa A, Hong SM, Heaphy CM, Singhi AD (2021) Non-functional pancreatic neuroendocrine tumours: ATRX/DAXX and alternative lengthening of telomeres (ALT) are prognostically independent from ARX/PDX1 expression and tumour size. Gut. https://doi.org/10.1136/gutjnl-2020-322595.
Asa SL, Ezzat S (2021) Hypothalamic hormone-producing tumors. Handb Clin Neurol 181: 67-74. https://doi.org/10.1016/B978-0-12-820683-6.00006-3.
Bellizzi AM (2020) SATB2 in neuroendocrine neoplasms: strong expression is restricted to well-differentiated tumours of lower gastrointestinal tract origin and is most frequent in Merkel cell carcinoma among poorly differentiated carcinomas. Histopathology 76: 251-264. https://doi.org/10.1111/his.13943.
Moshiri AS, Doumani R, Yelistratova L, Blom A, Lachance K, Shinohara MM, Delaney M, Chang O, McArdle S, Thomas H, Asgari MM, Huang ML, Schwartz SM, Nghiem P (2017) Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J Invest Dermatol 137: 819–827. S0022–202X(16)32613–6 [pii];https://doi.org/10.1016/j.jid.2016.10.028.
Asa SL (2021) Challenges in the Diagnosis of Pituitary Neuroendocrine Tumors. Endocr Pathol 32: 222-227. https://doi.org/10.1007/s12022-021-09678-x.
Hackeng WM, Schelhaas W, Morsink FHM, Heidsma CM, van ES, Valk GD, Vriens MR, Heaphy CM, Nieveen van Dijkum EJM, Offerhaus GJA, Dreijerink KMA, Brosens LAA (2020) Alternative Lengthening of Telomeres and Differential Expression of Endocrine Transcription Factors Distinguish Metastatic and Non-metastatic Insulinomas. Endocr Pathol 31: 108-118. https://doi.org/10.1007/s12022-020-09611-8.
Kim JY, Kim KS, Kim KJ, Park IJ, Lee JL, Myung SJ, Park Y, Park YS, Yu CS, Kim JC, Yu E, Jang HJ, Hong SM (2015) Non-L-cell immunophenotype and large tumor size in rectal neuroendocrine tumors are associated with aggressive clinical behavior and worse prognosis. Am J Surg Pathol 39: 632-643. https://doi.org/10.1097/PAS.0000000000000400.
Sohn JH, Cho MY, Park Y, Kim H, Kim WH, Kim JM, Jung ES, Kim KM, Lee JH, Chan HK, Park DY, Joo M, Kim S, Moon WS, Kang MS, Jin SY, Kang YK, Yoon SO, Han H, Choi E (2015) Prognostic Significance of Defining L-Cell Type on the Biologic Behavior of Rectal Neuroendocrine Tumors in Relation with Pathological Parameters. Cancer Res Treat 47: 813-822. https://doi.org/10.4143/crt.2014.238.
Lee SH, Kim BC, Chang HJ, Sohn DK, Han KS, Hong CW, Lee EJ, Lee JB, Lee DS, Lee IT, Youk EG (2013) Rectal neuroendocrine and L-cell tumors: diagnostic dilemma and therapeutic strategy. Am J Surg Pathol 37: 1044-1052. https://doi.org/10.1097/PAS.0b013e3182819f0f.
Xu B, Fuchs TL, Ahmadi S, Alghamdi M, Alzumaili B, Bani MA, Baudin E, Chou A, De LA, Fagin JA, Ganly I, Glover A, Hartl D, Kanaan C, Khneisser P, Najdawi F, Nigam A, Papachristos A, Repaci A, Spanheimer PM, Solaroli E, Untch BR, Barletta JA, Tallini G, Al GA, Gill AJ, Ghossein RA (2022) International Medullary Thyroid Carcinoma Grading System: A Validated Grading System for Medullary Thyroid Carcinoma. J Clin Oncol 40: 96-104. https://doi.org/10.1200/JCO.21.01329.
La Rosa S, Bonzini M, Sciarra A, Asioli S, Maragliano R, Arrigo M, Foschini MP, Righi A, Maletta F, Motolese A, Papotti M, Sessa F, Uccella S (2020) Exploring the Prognostic Role of Ki67 Proliferative Index in Merkel Cell Carcinoma of the Skin: Clinico-Pathologic Analysis of 84 Cases and Review of the Literature. Endocr Pathol 31: 392-400. https://doi.org/10.1007/s12022-020-09640-3.
Uccella S, La Rosa S, Metovic J, Marchiori D, Scoazec JY, Volante M, Mete O, Papotti M (2021) Genomics of High-Grade Neuroendocrine Neoplasms: Well-Differentiated Neuroendocrine Tumor with High-Grade Features (G3 NET) and Neuroendocrine Carcinomas (NEC) of Various Anatomic Sites. Endocr Pathol 32: 192-210. https://doi.org/10.1007/s12022-020-09660-z.
Liverani C, Bongiovanni A, Mercatali L, Pieri F, Spadazzi C, Miserocchi G, Di MG, Foca F, Ravaioli S, De VA, Cocchi C, Rossi G, Recine F, Ibrahim T (2021) Diagnostic and Predictive Role of DLL3 Expression in Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr Pathol 32: 309-317. https://doi.org/10.1007/s12022-020-09657-8.
Li B, Li X, Mao R, Liu M, Fu L, Shi L, Zhao S, Fu M (2021) Overexpression of ODF1 in Gastrointestinal Tract Neuroendocrine Neoplasms: a Novel Potential Immunohistochemical Biomarker for Well-differentiated Neuroendocrine Tumors. Endocr Pathol 32: 301-308. https://doi.org/10.1007/s12022-020-09649-8.
Konukiewitz B, Jesinghaus M, Steiger K, Schlitter AM, Kasajima A, Sipos B, Zamboni G, Weichert W, Pfarr N, Kloppel G (2018) Pancreatic neuroendocrine carcinomas reveal a closer relationship to ductal adenocarcinomas than to neuroendocrine tumors G3. Hum Pathol 77: 70–79. S0046–8177(18)30095–9; https://doi.org/10.1016/j.humpath.2018.03.018.
Kasajima A, Konukiewitz B, Schlitter AM, Weichert W, Kloppel G (2021) An analysis of 130 neuroendocrine tumors G3 regarding prevalence, origin, metastasis, and diagnostic features. Virchows Arch. https://doi.org/10.1007/s00428-021-03202-6.
Asa SL, La Rosa S, Basturk O, Adsay V, Minnetti M, Grossman AB (2021) Molecular Pathology of Well-Differentiated Gastro-entero-pancreatic Neuroendocrine Tumors. Endocr Pathol 32: 169-191. https://doi.org/10.1007/s12022-021-09662-5.
Volante M, Mete O, Pelosi G, Roden AC, Speel EJM, Uccella S (2021) Molecular Pathology of Well-Differentiated Pulmonary and Thymic Neuroendocrine Tumors: What Do Pathologists Need to Know? Endocr Pathol 32: 154-168. https://doi.org/10.1007/s12022-021-09668-z.
Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA (2012) Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 36: 173-184. https://doi.org/10.1097/PAS.0b013e3182417d36.
Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, Beras A, Spencer R, Lopardo J, Bodd F, Montecalvo J, Sauter JL, Chang JC, Buonocore DJ, Travis WD, Sen T, Poirier JT, Rudin CM, Rekhtman N (2020) SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J Thorac Oncol 15: 1823–1835. S1556–0864(20)30755–3; https://doi.org/10.1016/j.jtho.2020.09.009.
Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D, Massion PP, Minna JD, Oliver TG, Quaranta V, Sage J, Thomas RK, Vakoc CR, Gazdar AF (2019) Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19: 289-297. https://doi.org/10.1038/s41568-019-0133-9.
Cejas P, Xie Y, Font-Tello A, Lim K, Syamala S, Qiu X, Tewari AK, Shah N, Nguyen HM, Patel RA, Brown L, Coleman I, Hackeng WM, Brosens L, Dreijerink KMA, Ellis L, Alaiwi SA, Seo JH, Baca S, Beltran H, Khani F, Pomerantz M, Dall'Agnese A, Crowdis J, Van Allen EM, Bellmunt J, Morrisey C, Nelson PS, DeCaprio J, Farago A, Dyson N, Drapkin B, Liu XS, Freedman M, Haffner MC, Corey E, Brown M, Long HW (2021) Subtype heterogeneity and epigenetic convergence in neuroendocrine prostate cancer. Nat Commun 12: 5775. https://doi.org/10.1038/s41467-021-26042-z.
Chan CS, Laddha SV, Lewis PW, Koletsky MS, Robzyk K, Da SE, Torres PJ, Untch BR, Li J, Bose P, Chan TA, Klimstra DS, Allis CD, Tang LH (2018) ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 9: 4158. https://doi.org/10.1038/s41467-018-06498-2.
Cejas P, Drier Y, Dreijerink KMA, Brosens LAA, Deshpande V, Epstein CB, Conemans EB, Morsink FHM, Graham MK, Valk GD, Vriens MR, Castillo CF, Ferrone CR, Adar T, Bowden M, Whitton HJ, Da SA, Font-Tello A, Long HW, Gaskell E, Shoresh N, Heaphy CM, Sicinska E, Kulke MH, Chung DC, Bernstein BE, Shivdasani RA (2019) Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med 25: 1260-1265. https://doi.org/10.1038/s41591-019-0493-4.
Boons G, Vandamme T, Ibrahim J, Roeyen G, Driessen A, Peeters D, Lawrence B, Print C, Peeters M, Van CG, Op de BK (2020) PDX1 DNA Methylation Distinguishes Two Subtypes of Pancreatic Neuroendocrine Neoplasms with a Different Prognosis Cancers (Basel) 12. https://doi.org/10.3390/cancers12061461
Di Domenico A, Pipinikas CP, Maire RS, Brautigam K, Simillion C, Dettmer MS, Vassella E, Thirlwell C, Perren A, Marinoni I (2020) Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun Biol 3: 740. https://doi.org/10.1038/s42003-020-01479-y.
Dreijerink KM, Hackeng WM, Singhi AD, Heaphy CM, Brosens LA (2022) Clinical implications of cell-of-origin epigenetic characteristics in non-functional pancreatic neuroendocrine tumors. J Pathol 256: 143-148. https://doi.org/10.1002/path.5834.
Hong X, Qiao S, Li F, Wang W, Jiang R, Wu H, Chen H, Liu L, Peng J, Wang J, Jia C, Liang X, Dai H, Jiang J, Zhang T, Liao Q, Dai M, Cong L, Han X, Guo D, Liang Z, Li D, Zheng Z, Ye C, Li S, Zhao Y, Wu K, Wu W (2020) Whole-genome sequencing reveals distinct genetic bases for insulinomas and non-functional pancreatic neuroendocrine tumours: leading to a new classification system. Gut 69: 877-887. https://doi.org/10.1136/gutjnl-2018-317233.
Inzani F, Petrone G, Fadda G, Rindi G (2017) Cyto-histology in NET: what is necessary today and what is the future? Rev Endocr Metab Disord 18: 381-391. https://doi.org/10.1007/s11154-017-9428-x.
Tang LH, Klimstra DS (2011) Conundrums and Caveats in Neuroendocrine Tumors of the Pancreas. Surg Pathol Clin 4: 589–624. S1875–9181(11)00095-X; https://doi.org/10.1016/j.path.2011.03.003.
Tang LH (2020) Pancreatic Neuroendocrine Neoplasms: Landscape and Horizon. Arch Pathol Lab Med . https://doi.org/10.5858/arpa.2019-0654-RA.
Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, Liu X, Zhang L, Giordano TJ, Bellizzi AM, Chen JH, Shi C, Allen P, Reidy DL, Wolfgang CL, Saka B, Rezaee N, Deshpande V, Klimstra DS (2014) Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol 38: 437-447. https://doi.org/10.1097/PAS.0000000000000169.
Mete O, Asa SL (2020) Structure, Function, and Morphology in the Classification of Pituitary Neuroendocrine Tumors: the Importance of Routine Analysis of Pituitary Transcription Factors. Endocr Pathol 31: 330-336. https://doi.org/10.1007/s12022-020-09646-x.
Mete O, Cintosun A, Pressman I, Asa SL (2018) Epidemiology and biomarker profile of pituitary adenohypophysial tumors. Mod Pathol 31: 900-909. https://doi.org/10.1038/s41379-018-0016-8.
Gomez-Hernandez K, Ezzat S, Asa SL, Mete O (2015) Clinical Implications of Accurate Subtyping of Pituitary Adenomas: Perspectives from the Treating Physician. Turk Patoloji Derg 31 Suppl 1: 4-17. https://doi.org/10.5146/tjpath.2015.01311.
Mete O, Asa SL (2013) Therapeutic implications of accurate classification of pituitary adenomas. Semin Diagn Pathol 30: 158-164.
Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM, Paik PK, Drilon AE, Socci N, Poirier JT, Shen R, Berger MF, Moreira AL, Travis WD, Rudin CM, Ladanyi M (2016) Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res 22: 3618-3629. https://doi.org/10.1158/1078-0432.CCR-15-2946.
Quinn AM, Chaturvedi A, Nonaka D (2017) High-grade Neuroendocrine Carcinoma of the Lung With Carcinoid Morphology: A Study of 12 Cases. Am J Surg Pathol 41: 263-270. https://doi.org/10.1097/PAS.0000000000000767.
Marchio C, Gatti G, Massa F, Bertero L, Filosso P, Pelosi G, Cassoni P, Volante M, Papotti M (2017) Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch 471: 713-720. https://doi.org/10.1007/s00428-017-2177-0.
Kasajima A, Konukiewitz B, Oka N, Suzuki H, Sakurada A, Okada Y, Kameya T, Ishikawa Y, Sasano H, Weichert W, Kloppel G (2019) Clinicopathological Profiling of Lung Carcinoids with a Ki67 Index > 20. Neuroendocrinology 108: 109-120. https://doi.org/10.1159/000495806.
Saeger W, Mawrin C, Meinhardt M, Wefers AK, Jacobsen F (2021) Two Pituitary Neuroendocrine Tumors (PitNETs) with Very High Proliferation and TP53 Mutation - High-Grade PitNET or PitNEC? Endocr Pathol . https://doi.org/10.1007/s12022-021-09693-y.
Kim JA, Choi WH, Kim CN, Moon YS, Chang SH, Lee HR (2011) Duodenal somatostatinoma: a case report and review. Korean J Intern Med 26: 103-107. https://doi.org/10.3904/kjim.2011.26.1.103.
Asa SL, Arkun K, Tischler AS, Qamar A, Deng FM, Perez-Ordonez B, Weinreb I, Bishop JA, Wenig BM, Mete O (2021) Middle Ear “Adenoma”: a Neuroendocrine Tumor with Predominant L Cell Differentiation. Endocr Pathol 32: 433-441. https://doi.org/10.1007/s12022-021-09684-z.
Chan ES, Alexander J, Swanson PE, Jain D, Yeh MM (2012) PDX-1, CDX-2, TTF-1, and CK7: a reliable immunohistochemical panel for pancreatic neuroendocrine neoplasms. Am J Surg Pathol 36: 737-743. https://doi.org/10.1097/PAS.0b013e31824aba59.
Singh S, Asa SL, Dey C, Kennecke H, Laidley D, Law C, Asmis T, Chan D, Ezzat S, Goodwin R, Mete O, Pasieka J, Rivera J, Wong R, Segelov E, Rayson D (2016) Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev 47: 32–45. S0305–7372(16)30021–4. https://doi.org/10.1016/j.ctrv.2016.05.003.
Rooper LM, Sharma R, Li QK, Illei PB, Westra WH (2017) INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am J Surg Pathol 41: 1561-1569. https://doi.org/10.1097/PAS.00000000000009.
Rooper LM, Bishop JA, Westra WH (2018) INSM1 is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am J Surg Pathol 42: 665-671. https://doi.org/10.1097/PAS.0000000000001037.
Kim IE, Jr., Amin A, Wang LJ, Cheng L, Perrino CM (2020) Insulinoma-associated Protein 1 (INSM1) Expression in Small Cell Neuroendocrine Carcinoma of the Urinary Tract. Appl Immunohistochem Mol Morphol 28: 687-693. https://doi.org/10.1097/PAI.0000000000000824.
Juhlin CC, Zedenius J, Hoog A (2020) Clinical Routine Application of the Second-generation Neuroendocrine Markers ISL1, INSM1, and Secretagogin in Neuroendocrine Neoplasia: Staining Outcomes and Potential Clues for Determining Tumor Origin. Endocr Pathol 31: 401-410. https://doi.org/10.1007/s12022-020-09645-y.
Kovari B, Turkevi-Nagy S, Bathori A, Fekete Z, Krenacs L (2020) Syntaxin 1: A Novel Robust Immunophenotypic Marker of Neuroendocrine Tumors Int J Mol Sci 21.https://doi.org/10.3390/ijms21041213
Uccella S, La Rosa S (2020) Looking into digestive mixed neuroendocrine - nonneuroendocrine neoplasms: subtypes, prognosis, and predictive factors. Histopathology 77: 700-717. https://doi.org/10.1111/his.14178.
Tang LH, Basturk O, Sue JJ, Klimstra DS (2016) A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol 40: 1192-1202. https://doi.org/10.1097/PAS.0000000000000662.
Maragliano R, Libera L, Carnevali I, Pensotti V, De VG, Testa M, Amaglio C, Leoni E, Formenti G, Sessa F, Furlan D, Uccella S (2021) Mixed Neuroendocrine/Non-neuroendocrine Neoplasm (MiNEN) of the Ovary Arising from Endometriosis: Molecular Pathology Analysis in Support of a Pathogenetic Paradigm. Endocr Pathol. https://doi.org/10.1007/s12022-021-09689-8.
Uccella S, Ottini G, Facco C, Maragliano R, Asioli S, Sessa F, La Rosa S (2017) Neuroendocrine neoplasms of the head and neck and olfactory neuroblastoma. Diagnosis and classification. Pathologica 109: 14–30. Pacini.
Zhang C, Schmidt LA, Hatanaka K, Thomas D, Lagstein A, Myers JL (2014) Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am J Clin Pathol 142: 320-324. https://doi.org/10.1309/AJCPGA0IUA8BHQEZ.
Lewis JS, Jr., Chernock RD, Bishop JA (2018) Squamous and Neuroendocrine Specific Immunohistochemical Markers in Head and Neck Squamous Cell Carcinoma: A Tissue Microarray Study. Head Neck Pathol 12: 62-70. https://doi.org/10.1007/s12105-017-0825-y.
Kervarrec T, Tallet A, Miquelestorena-Standley E, Houben R, Schrama D, Gambichler T, Berthon P, Le CY, Hainaut-Wierzbicka E, Aubin F, Bens G, Tabareau-Delalande F, Beneton N, Fromont G, Arbion F, Leteurtre E, Touze A, Samimi M, Guyetant S (2019) Diagnostic accuracy of a panel of immunohistochemical and molecular markers to distinguish Merkel cell carcinoma from other neuroendocrine carcinomas. Mod Pathol 32: 499-510. https://doi.org/10.1038/s41379-018-0155-y.
Inzani F, Petrone G, Rindi G (2018) The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am 47: 463–470. S0889–8529(18)30520–6; https://doi.org/10.1016/j.ecl.2018.04.008.
Xue Y, Reid MD, Pehlivanoglu B, Obeng RC, Jiang H, Memis B, Lui SK, Sarmiento J, Kooby D, Maithel SK, El-Rayes B, Basturk O, Adsay V (2020) Morphologic Variants of Pancreatic Neuroendocrine Tumors: Clinicopathologic Analysis and Prognostic Stratification. Endocr Pathol 31: 239-253. https://doi.org/10.1007/s12022-020-09628-z.
Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, Sigel C, Klimstra DS (2015) The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 39: 683-690. https://doi.org/10.1097/PAS.0000000000000408.
Dinter H, Bohnenberger H, Beck J, Bornemann-Kolatzki K, Schutz E, Kuffer S, Klein L, Franks TJ, Roden A, Emmert A, Hinterthaner M, Marino M, Brcic L, Popper H, Weis CA, Pelosi G, Marx A, Strobel P (2019) Molecular Classification of Neuroendocrine Tumors of the Thymus. J Thorac Oncol 14: 1472–1483. S1556–0864(19)30312–0; https://doi.org/10.1016/j.jtho.2019.04.015.
Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS (2016) Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res 22: 1011–1017. 1078–0432.CCR-15–0548.
La Rosa S (2021) Challenges in High-grade Neuroendocrine Neoplasms and Mixed Neuroendocrine/Non-neuroendocrine Neoplasms. Endocr Pathol 32: 245-257. https://doi.org/10.1007/s12022-021-09676-z.
George J, Lim JS, Jang SJ, Cun Y, Ozretic L, Kong G, Leenders F, Lu X, Fernandez-Cuesta L, Bosco G, Muller C, Dahmen I, Jahchan NS, Park KS, Yang D, Karnezis AN, Vaka D, Torres A, Wang MS, Korbel JO, Menon R, Chun SM, Kim D, Wilkerson M, Hayes N, Engelmann D, Putzer B, Bos M, Michels S, Vlasic I, Seidel D, Pinther B, Schaub P, Becker C, Altmuller J, Yokota J, Kohno T, Iwakawa R, Tsuta K, Noguchi M, Muley T, Hoffmann H, Schnabel PA, Petersen I, Chen Y, Soltermann A, Tischler V, Choi CM, Kim YH, Massion PP, Zou Y, Jovanovic D, Kontic M, Wright GM, Russell PA, Solomon B, Koch I, Lindner M, Muscarella LA, la TA, Field JK, Jakopovic M, Knezevic J, Castanos-Velez E, Roz L, Pastorino U, Brustugun OT, Lund-Iversen M, Thunnissen E, Kohler J, Schuler M, Botling J, Sandelin M, Sanchez-Cespedes M, Salvesen HB, Achter V, Lang U, Bogus M, Schneider PM, Zander T, Ansen S, Hallek M, Wolf J, Vingron M, Yatabe Y, Travis WD, Nurnberg P, Reinhardt C, Perner S, Heukamp L, Buttner R, Haas SA, Brambilla E, Peifer M, Sage J, Thomas RK (2015) Comprehensive genomic profiles of small cell lung cancer. Nature 524: 47-53. https://doi.org/10.1038/nature14664.
Takizawa N, Ohishi Y, Hirahashi M, Takahashi S, Nakamura K, Tanaka M, Oki E, Takayanagi R, Oda Y (2015) Molecular characteristics of colorectal neuroendocrine carcinoma; similarities with adenocarcinoma rather than neuroendocrine tumor. Hum Pathol 46: 1890–1900. S0046–8177(15)00310-X; https://doi.org/10.1016/j.humpath.2015.08.006.
La Rosa S, Sessa F, Uccella S (2016) Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol 27: 284-311. https://doi.org/10.1007/s12022-016-9432-9.
Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, Vanoli A, Tagliabue G, Pisa E, Messerini L, Centonze G, Inzani F, Scarpa A, Papotti M, Volante M, Sessa F, Fazio N, Pruneri G, Rindi G, Solcia E, La Rosa S, Capella C (2018) Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer 25: 583-593. https://doi.org/10.1530/ERC-17-0557.
Milione M, Maisonneuve P, Grillo F, Mangogna A, Centonze G, Prinzi N, Pusceddu S, Garzone G, Cattaneo L, Busico A, Bossi P, Spaggiari P, Pellegrinelli A, Del GA, Ferrero S, Kankava K, Pruneri G, Rolli L, Roca E, Bercich L, Tironi A, Benvenuti MR, Gallazzi MS, Romano R, Berruti A, Pastorino U, Capella C (2021) Ki-67 Index of 55% Distinguishes Two Groups of Bronchopulmonary Pure and Composite Large Cell Neuroendocrine Carcinomas with Distinct Prognosis. Neuroendocrinology 111: 475-489. https://doi.org/10.1159/000508376.
Horn LC, Hentschel B, Bilek K, Richter CE, Einenkel J, Leo C (2006) Mixed small cell carcinomas of the uterine cervix: prognostic impact of focal neuroendocrine differentiation but not of Ki-67 labeling index. Ann Diagn Pathol 10: 140–143. S1092–9134(05)00125–5; https://doi.org/10.1016/j.anndiagpath.2005.07.019 .
Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG (2020) Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis J Clin Med 9.https://doi.org/10.3390/jcm9010273
Cordier R (1924) Les cellules argentaffines dans les tumeurs intestinales. Arch Int Med Exp 5.
Lewin K (1987) Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol 11 Suppl 1: 71-86. https://doi.org/10.1097/00000478-198700111-00007.
Capella C, La Rosa S, Uccella S, Billo P, Cornaggia M (2000) Mixed endocrine-exocrine tumors of the gastrointestinal tract. Semin Diagn Pathol 17: 91-103.
La Rosa S, Marando A, Sessa F, Capella C (2012) Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel) 4: 11-30. https://doi.org/10.3390/cancers4010011.
de ML, Cros J, Neuzillet C, Hentic O, Egal A, Muller N, Bouche O, Cadiot G, Ruszniewski P, Couvelard A, Hammel P (2017) Digestive System Mixed Neuroendocrine-Non-Neuroendocrine Neoplasms. Neuroendocrinology 105: 412-425. https://doi.org/10.1159/000475527.
Chejfec G, Capella C, Solcia E, Jao W, Gould VE (1985) Amphicrine cells, dysplasias, and neoplasias. Cancer 56: 2683-2690. https://doi.org/10.1002/1097-0142(19851201)56.112683/aid-cncr28205611273.0.co2-l.
Mandoky L (1999) Amphicrine tumor. Pathol Oncol Res 5: 239-244. https://doi.org/10.1053/paor.1999.0211.
Bellur S, van der Kwast T, Mete O (2019) Evolving concepts in prostatic neuroendocrine manifestations: from focal divergent differentiation to amphicrine carcinoma. Hum Pathol 85: 313–327. S0046–8177(18)30468–4, https://doi.org/10.1016/j.humpath.2018.11.016.
Huang D, Ren F, Ni S, Tan C, Weng W, Zhang M, Xu M, Wang L, Xu Q, Sheng W (2019) Amphicrine carcinoma of the stomach and intestine: a clinicopathologic and pan-cancer transcriptome analysis of a distinct entity. Cancer Cell Int 19: 310. https://doi.org/10.1186/s12935-019-1031-7.
(2017) TNM Classification of Malignant Tumours. Oxford: Wiley Blackwell.
O Casar-Borota HB Boldt BE Engstrom MS Andersen B Baussart D Bengtsson K Berinder B Ekman U Feldt-Rasmussen C Hoybye JOL Jorgensen AJ Kolnes M Korbonits AK Rasmussen JR Lindsay PB Loughrey D Maiter E Manojlovic-Gacic J Pahnke PL Poliani V Popovic O Ragnarsson C Schalin-Jantti D Scheie M Toth C Villa M Wirenfeldt J Kunicki P Burman 2020 Corticotroph aggressive pituitary tumours and carcinomas frequently harbour ATRX mutations J Clin Endocrinol Metab. 5940659https://doi.org/10.1210/clinem/dgaa749
Luchini C, Pelosi G, Scarpa A, Mattiolo P, Marchiori D, Maragliano R, Sessa F, Uccella S (2021) Neuroendocrine neoplasms of the biliary tree, liver and pancreas: a pathological approach. Pathologica 113: 28–38. https://doi.org/10.32074/1591-951X-231.
Hodgson A, Pakbaz S, Tayyari F, Young JEM, Mete O (2019) Diagnostic Pitfall: Parathyroid Carcinoma Expands the Spectrum of Calcitonin and Calcitonin Gene-Related Peptide Expressing Neuroendocrine Neoplasms. Endocr Pathol 30: 168-172. https://doi.org/10.1007/s12022-019-9572-9.
Nozieres C, Chardon L, Goichot B, Borson-Chazot F, Hervieu V, Chikh K, Lombard-Bohas C, Walter T (2016) Neuroendocrine tumors producing calcitonin: characteristics, prognosis and potential interest of calcitonin monitoring during follow-up. Eur J Endocrinol 174: 335-341. https://doi.org/10.1530/EJE-15-0917.
Mohanty SK, Kim SA, DeLair DF, Bose S, Laury AR, Chopra S, Mertens RB, Dhall D (2016) Comparison of metastatic neuroendocrine neoplasms to the breast and primary invasive mammary carcinomas with neuroendocrine differentiation. Mod Pathol 29: 788-798. https://doi.org/10.1038/modpathol.2016.69.
Mete O, Asa SL (2013) Precursor lesions of endocrine system neoplasms. Pathol 45: 316-330.
Klimstra DS, Adsay V (2016) Acinar neoplasms of the pancreas-A summary of 25 years of research. Semin Diagn Pathol 33: 307–318. S0740–2570(16)30037–5; https://doi.org/10.1053/j.semdp.2016.05.009.
Nguyen NQ, Johns AL, Gill AJ, Ring N, Chang DK, Clarkson A, Merrett ND, Kench JG, Colvin EK, Scarlett CJ, Biankin AV (2011) Clinical and immunohistochemical features of 34 solid pseudopapillary tumors of the pancreas. J Gastroenterol Hepatol 26: 267-274. https://doi.org/10.1111/j.1440-1746.2010.06466.x.
Creytens D (2020) SATB2 and TLE1 Expression in BCOR-CCNB3 (Ewing-like) Sarcoma, Mimicking Small Cell Osteosarcoma and Poorly Differentiated Synovial Sarcoma. Appl Immunohistochem Mol Morphol 28: e10-e12. https://doi.org/10.1097/PAI.0000000000000601.
Huang Q, Wu H, Nie L, Shi J, Lebenthal A, Chen J, Sun Q, Yang J, Huang L, Ye Q (2013) Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol 37: 467-483. https://doi.org/10.1097/PAS.0b013e31826d2639.
Rekhtman N, Montecalvo J, Chang JC, Alex D, Ptashkin RN, Ai N, Sauter JL, Kezlarian B, Jungbluth A, Desmeules P, Beras A, Bishop JA, Plodkowski AJ, Gounder MM, Schoenfeld AJ, Namakydoust A, Li BT, Rudin CM, Riely GJ, Jones DR, Ladanyi M, Travis WD (2020) SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J Thorac Oncol 15: 231–247. S1556–0864(19)33643–3 ;https://doi.org/10.1016/j.jtho.2019.10.023.
Auguste A, Blanc-Durand F, Deloger M, Le FA, Bareja R, Wilkes DC, Richon C, Brunn B, Caron O, Devouassoux-Shisheboran M, Gouy S, Morice P, Bentivegna E, Sboner A, Elemento O, Rubin MA, Pautier P, Genestie C, Cyrta J, Leary A (2020) Small Cell Carcinoma of the Ovary, Hypercalcemic Type (SCCOHT) beyond SMARCA4 Mutations: A Comprehensive Genomic Analysis Cells 9.https://doi.org/10.3390/cells9061496
Mamilla D, Manukyan I, Fetsch PA, Pacak K, Miettinen M (2020) Immunohistochemical distinction of paragangliomas from epithelial neuroendocrine tumors-gangliocytic duodenal and cauda equina paragangliomas align with epithelial neuroendocrine tumors. Hum Pathol 103: 72-82. https://doi.org/10.1016/j.humpath.2020.07.010.
Bockmayr M, Korner M, Schweizer L, Schuller U (2021) Cauda equina paragangliomas express HOXB13. Neuropathol Appl Neurobiol 47: 889-890. https://doi.org/10.1111/nan.12713.
Greenberg SE, Jacobs MF, Wachtel H, Anson A, Buchmann L, Cohen DL, Bonanni M, Bennett B, Naumer A, Schaefer AM, Kohlmann W, Nathanson KL, Else T, Fishbein L (2020) Tumor detection rates in screening of individuals with SDHx-related hereditary paraganglioma-pheochromocytoma syndrome. Genet Med 22: 2101-2107. https://doi.org/10.1038/s41436-020-0921-3.
Okubo Y, Yokose T, Motohashi O, Miyagi Y, Yoshioka E, Suzuki M, Washimi K, Kawachi K, Nito M, Nemoto T, Shibuya K, Kameda Y (2016) Duodenal Rare Neuroendocrine Tumor: Clinicopathological Characteristics of Patients with Gangliocytic Paraganglioma. Gastroenterol Res Pract 2016: 5257312. https://doi.org/10.1155/2016/5257312
Torske KR, Thompson LD (2002) Adenoma versus carcinoid tumor of the middle ear: a study of 48 cases and review of the literature. Mod Pathol 15: 543-555. https://doi.org/10.1038/modpathol.3880561
Ketabchi S, Massi D, Franchi A, Vannucchi P, Santucci M (2001) Middle ear adenoma is an amphicrine tumor: why call it adenoma? Ultrastruct Pathol 25: 73-78. https://doi.org/10.1080/019131201300004717
Bell D, El-Naggar AK, Gidley PW (2017) Middle ear adenomatous neuroendocrine tumors: a 25-year experience at MD Anderson Cancer Center. Virchows Arch 471: 667-672. https://doi.org/10.1007/s00428-017-2155-6
Reubi JC, Lamberts SW, Maurer R (1988) Somatostatin receptors in normal and tumoral tissue. Horm Res 29: 65-69.
Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W, Esposito I, Pfarr N, Kloppel G (2017) Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol 30: 587-598. https://doi.org/10.1038/modpathol.2016.217.
Di Stasio GD, Buonomano P, Travaini LL, Grana CM, Mansi L (2021) From the Magic Bullet to Theragnostics: Certitudes and Hypotheses, Trying to Optimize the Somatostatin Model Cancers (Basel) 13.https://doi.org/10.3390/cancers13143474
Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, Cwikla J, Baum RP, Kwekkeboom DJ, Paganelli G, Krenning EP, Modlin IM (2018) PRRT genomic signature in blood for prediction of (177)Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging 45: 1155-1169. https://doi.org/10.1007/s00259-018-3967-6.
Vanoli A, La Rosa S, Klersy C, Grillo F, Albarello L, Inzani F, Maragliano R, Manca R, Luinetti O, Milione M, Doglioni C, Rindi G, Capella C, Solcia E (2017) Four Neuroendocrine Tumor Types and Neuroendocrine Carcinoma of the Duodenum: Analysis of 203 Cases. Neuroendocrinology 104: 112-125. https://doi.org/10.1159/000444803.
Kasajima A, Papotti M, Ito W, Brizzi MP, La SA, Rapa I, Tachibana T, Yazdani S, Sasano H, Volante M (2018) High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum Pathol 72: 144–152. S0046–8177(17)30427–6 ; https://doi.org/10.1016/j.humpath.2017.11.008.
Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, De RG, Dogliotti L, Colao A, Papotti M (2007) Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20: 1172-1182. https://doi.org/10.1038/modpathol.3800954.
Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, Tsung A, Marsh JW, Lee KK, Hogg ME, Bahary N, Brand RE, McGrath KM, Slivka A, Cressman KL, Fuhrer K, O'Sullivan RJ (2017) Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin Cancer Res 23: 600-609. https://doi.org/10.1158/1078-0432.CCR-16-1113.
Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, Hunger F, Pasquinelli S, Speel EJ, Perren A (2014) Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 146: 453-460.
Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardiere C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH, Cabanillas ME (2020) Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 383: 825-835. https://doi.org/10.1056/NEJMoa2005651.
Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, Kobayashi N, Ikeda M, Ito T, Nakamori S, Ishii H, Kodama Y, Morizane C, Okusaka T, Yanagimoto H, Notohara K, Taguchi H, Kitano M, Yane K, Maguchi H, Tsuchiya Y, Komoto I, Tanaka H, Tsuji A, Hashigo S, Kawaguchi Y, Mine T, Kanno A, Murohisa G, Miyabe K, Takagi T, Matayoshi N, Yoshida T, Hara K, Imamura M, Furuse J, Yatabe Y, Mizuno N (2017) Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res 23: 4625-4632. https://doi.org/10.1158/1078-0432.CCR-16-3135.
Derks JL, Leblay N, Thunnissen E, van Suylen RJ, den BM, Groen HJM, Smit EF, Damhuis R, van den Broek EC, Charbrier A, Foll M, McKay JD, Fernandez-Cuesta L, Speel EM, Dingemans AC (2018) Molecular Subtypes of Pulmonary Large-cell Neuroendocrine Carcinoma Predict Chemotherapy Treatment Outcome. Clin Cancer Res 24: 33-42. https://doi.org/10.1158/1078-0432.CCR-17-1921.
Lacombe C, RO De A, Couvelard A, Turpin A, Cazes O, Hentic V, Gounant G, Zalcman P, Ruszniewski J, Cros de ML (2021) Biomarkers of Response to Etoposide-Platinum Chemotherapy in Patients with Grade 3 Neuroendocrine Neoplasms Cancers (Basel) 13.https://doi.org/10.3390/cancers13040643
Duan K, Mete O (2017) Familial endocrine tumor syndromes: Clinical and predictive roles of molecular histopathology. AJSP: Reviews and Reports 22: 246–268.
Gucer H, Szentgyorgyi E, Ezzat S, Asa SL, Mete O (2013) Inhibin-expressing clear cell neuroendocrine tumor of the ampulla: an unusual presentation of von Hippel-Lindau disease. Virchows Arch 463: 593-597.
Favier J, Meatchi T, Robidel E, Badoual C, Sibony M, Nguyen AT, Gimenez-Roqueplo AP, Burnichon N (2020) Carbonic anhydrase 9 immunohistochemistry as a tool to predict or validate germline and somatic VHL mutations in pheochromocytoma and paraganglioma-a retrospective and prospective study. Mod Pathol 33: 57-64. https://doi.org/10.1038/s41379-019-0343-4.
Mete O, Pakbaz S, Lerario AM, Giordano TJ, Asa SL (2021) Significance of Alpha-inhibin Expression in Pheochromocytomas and Paragangliomas. Am J Surg Pathol. https://doi.org/10.1097/PAS.0000000000001715.
Sekine R, Shimazu K, Nakano D, Yamaguchi T, Suzuki Y, Fukuda K, Yoshida T, Taguchi D, Iijima K, Nanjyo H, Shibata H (2022) A novel Lynch syndrome pedigree bearing germ-line MSH2 missense mutation c.1808A>T (Asp603Val). Jpn J Clin Oncol 52: 81–85. 6425237; https://doi.org/10.1093/jjco/hyab173.
Karamurzin Y, Zeng Z, Stadler ZK, Zhang L, Ouansafi I, Al-Ahmadie HA, Sempoux C, Saltz LB, Soslow RA, O'Reilly EM, Paty PB, Coit DG, Shia J, Klimstra DS (2012) Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol 43: 1677–1687. S0046–8177(11)00518–1; https://doi.org/10.1016/j.humpath.2011.12.012.
Karamurzin Y, Zeng Z, Stadler ZK, Zhang L, Ouansafi I, Al Ahmadie HA, Sempoux C, Saltz LB, Soslow RA, O'Reilly EM, Paty PB, Coit DG, Shia J, Klimstra DS (2012) Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol 43: 1677-1687.
Serracant BA, Serra PS, Blazquez Mana CM, Salas RC, Garcia MN, Bejarano GN, Romaguera MA, Andreu Navarro FJ, Bella Cueto MR, Borobia FG (2017) Pancreatic non-functioning neuroendocrine tumor: a new entity genetically related to Lynch syndrome. J Gastrointest Oncol 8: E73-E79. https://doi.org/10.21037/jgo.2017.07.02/jgo-08-05-E73.
Author information
Authors and Affiliations
Contributions
Conception and design: GR, OM, SLA; data collection and analysis: all authors; manuscript preparation and editing: all authors; approval of final manuscript: all authors.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
All authors consent to publication.
Competing Interests
Dr. Guido Rindi has had speaker engagements for the Advanced Accelerator Applications (AAA), a Novartis company. Dr. Shereen Ezzat has had speaker engagements for Novartis and Ipsen. Dr. David Klimstra is the Chief Medical officer (employee and equity holder) of Paige.AI. Dr. Ozgur Mete is the Editor-in-Chief of Endocrine Pathology. An independent senior editor handled this article and peer reviewed as per the journal standards. The remaining authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rindi, G., Mete, O., Uccella, S. et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol 33, 115–154 (2022). https://doi.org/10.1007/s12022-022-09708-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-022-09708-2
Keywords
- Grade 3 neuroendocrine tumor
- Neuroendocrine tumor
- WHO classification
- Neuroendocrine carcinoma
- MiNEN
- Amphicrine carcinoma
- PRRT
- Biomarkers
- CoGNET
- Cauda equina neuroendocrine tumor
- Paraganglioma
- Inherited neuroendocrine neoplasia
- Biomarkers
- INSM1
- Pancreas
- Merkel cell carcinoma
- Pituitary neuroendocrine tumor
- Medullary thyroid carcinoma
- L cell
- Middle ear neuroendocrine tumor