Abstract
Purpose
Endocrine disruptors (EDs) are exogenous substances able to impair endocrine system; consequently, they may cause numerous adverse effects. Over the last years, particular focus has been given to their harmful effects on reproductive system, but very little is known, especially in males. The aim of this review is to discuss the detrimental effects of EDs exposure on fetal testis development, male puberty, and transition age.
Methods
A search for the existing literature focusing on the impact of EDs on fetal testis development, male puberty, andrological parameters (anogenital distance, penile length, and testicular volume), and testicular cancer with particular regard to pubertal age provided the most current information available for this review. Human evidence-based reports were given priority over animal and in vitro experimental results. Given the paucity of available articles on this subject, all resources were given careful consideration.
Results
Information about the consequences associated with EDs exposure in the current literature is limited and often conflicting, due to the scarcity of human studies and their heterogeneity.
Conclusions
We conclude that current evidence does not clarify the impact of EDs on human male reproductive health, although severe harmful effects had been reported in animals. Despite controversial results, overall conclusion points toward a positive association between exposure to EDs and reproductive system damage. Further long-term studies performed on wide number of subjects are necessary in order to identify damaging compounds and remove them from the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the WHO/IPCS 2002 definition, “an Endocrine Disruptor (ED) is an exogenous substance or mixture that alters functions of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny.” Over the past two decades, public health has focused on the identification of environmental EDs that are able to adversely affect hormonal function [1]. EDs mimic naturally occurring hormones like estrogens and androgens and exert their toxicity by interfering with the normal hormonal homeostatic mechanisms that promote growth and development of tissues. EDs usually interfere with the hormonal binding to the corresponding receptor, notably the androgen receptor (AR) or the estrogen receptor. Subsequently, EDs can trigger two types of response: agonistic and/or antagonistic effect. In addition, recent discoveries in molecular biology confirmed a possible interference by several compounds with the cell cycle, the apoptotic mechanisms, and the epigenetic regulation [2]. Epigenetic changes are able to modify activation and expression of genes, though not altering the genetic code sequence. Changes in DNA methylation, histone modifications, and noncoding RNAs are involved. Recent studies reported a transmission of epigenetic shifts from father to child, suggesting a transgenerational inheritance [3, 4]. Therefore, EDs may have negative effects not only in exposed individuals, but also in their offspring and in future generations. Main EDs characteristics are summarized in Table 1. In addition to exposure by direct contact with these materials, EDs are also released into the environment. Therefore, exposure may occur through food and water consumption, inhalation, or dermal contact. During fetal and neonatal life it could also occur through placenta and breast feeding. As a consequence, EDs may affect human pre and postnatal development. In fact, infants can be affected already at prenatal level due to maternal exposure to EDs [5]. The effects of EDs on the male reproductive system are usually attributed to the interactions of these chemicals with the normal production and/or function of steroid hormones that are responsible for the masculinization of the Wolffian ducts [6]. In males, reproductive disorders associated with impaired fetal testis development or function vary in both phenotype and time of manifestation. Often gathered under the “testicular dysgenesis syndrome” (TDS) hypothesis [7], these male disorders range from hypospadias and cryptorchidism in infants [8,9,10,11] to low testosterone levels, infertility, and testicular cancer (TC) in adult men [12,13,14]. TDS has been correlated with environmental factors during fetal life [7].
The aim of this review is to discuss the detrimental effects of EDs exposure on fetal testis development, male puberty, and transition age, the latter defined as age 18–25 years. This definition is consistent with the recent discoveries in the field of neurophysiology, in fact a maturation of prefrontal cortex until 25 years old has been pointed out [15, 16]. This review aims to provide an overview of animal, in vitro, and human studies, though human evidence-based reports were given priority. In particular, andrological parameters of male health such as anogenital distance (AGD), penile length, and testicular volume (TV) were investigated.
Effects of EDs on testis development
Testis development during fetal life is crucial for male reproductive function in adulthood [17]. Indeed, fetal period is critical for the regular development of the testis and is known as a period of high sensitivity to many EDs [18]. Both functions of testis (spermatogenesis and steroidogenesis) are set up early during fetal life [19]. Primordial gonads appear between the 4th and the 6th week post fertilization. They are rapidly colonized by primordial germ cells that migrate from extra-embryonic areas [20]. Six weeks post fertilization, the differentiation of the testis is due to Sertoli cells surrounding testicular cords [21]. Sertoli cells are central for germ cell development [22] and Leydig cells differentiation [23]. Indeed, at 6th week post fertilization, fetal Leydig cells start producing testosterone and insulin-like factor 3 (INSL3). Both hormones are involved in testicular descent [24]. Furthermore, testosterone is crucial for fetal masculinization. Therefore, perturbations to the function of fetal Leydig cells can predispose to the development of male reproductive disorders including TDS and other disorders of sex development [25].
In the last years, literature focused on possible association between EDs and testis development. Several experimental animal studies were conducted to investigate a possible correlation, especially in mouse models. Di-2-ethylhexyl phthalate (DEHP) and di-n-butyl phthalate (DBP) are the most abundant phthalates which, after ingestion, are hydrolyzed into the active monoesters monoethylhexyl phthalate (MEHP) and mono-n-butyl phthalate (MBP), respectively. In rodents, the effects of DEHP and DBP exposure in utero have been largely described. Numerous studies reported a disruption of normal fetal testis development and the subsequent development of male reproductive disorders [17, 26]. It is interesting to note that in mice phthalates can induce a positive effect on testosterone secretion by the cultured fetal testis [27, 28]. Surprisingly, such effect was not observed in the human testis. In this regard, both organ cultures and xenograft experiments revealed no marked change in testosterone production [29,30,31], suggesting that exposure to environmental levels of DBP and DEHP is unlikely to result in effects on fetal testosterone production in humans. Notably, although no effects of phthalate exposure have been demonstrated in human fetal testes, antiandrogenic effects occur in adult human testis following in vitro culture. The different effect of exposure could depend on the developmental stage of the testis [32]. However, experimental studies in rodents and human fetal tissues are consistent regarding germ cells, showing a reduction in the gonocyte number following phthalate exposure [30, 33]. Concerning other EDs, inconsistent results on testosterone production and germ cell development have been identified in animal studies investigating the effects of BPA exposure on fetal testis development [34, 35]. In the same way, inconsistent results on association between BPA and clinical indicators of reduced fetal testosterone (cryptorchidism and hypospadias) have been reported in epidemiological studies [36, 37]. For human testicular tissue experiments, in vitro studies indicate the potential for BPA to reduce testosterone production in the fetal testicle, whereas xenotransplantation studies failed to demonstrate similar effects [34, 35]. In addition, in vivo human BPA exposure might be below the concentrations used for experimental studies involving animal or human tissues [38]. Regarding other EDs, PCBs have been associated with abnormal urogenital development in animal models [39, 40]. In particular, lactational exposure seems to affect histology of rat testis in both prepuberal and puberal F1 progeny [41]. In rats, perfluorooctanoic acid (PFOA) does not seem to affect fetal Sertoli cells but may increase tendency of apoptosis in fetal Leydig cells [42]. This damage seems to affect both proliferation and differentiation of stem Leydig cells or their progeny [43]. Regarding perfluorooctanesulfonic acid (PFOS), it seems to damage Sertoli cells by perturbing actin cytoskeleton in primary cultures of rodent and human [44, 45] and may directly inhibit pubertal development of rat Leydig cells [46]. In humans, prenatal PFOS exposure may increase fetal steroid hormone production, although no association with cryptorchidism or hypospadias has been observed [47]. Anti-Müllerian hormone and INSL3 have been recently recognized as optimal markers of Sertoli and Leydig cells function, respectively, in particular during the first years of life [48]. However, modifications of their levels after EDs exposure have not been widely investigated in human studies. These reports may eventually provide additional insight on the pathophysiology of endocrine disruption on testis development.
Effects of EDs on timing of puberty
The term puberty means a complex of psycho-neuro-endocrine changes that occur between the end of the childhood and the achievement of the complete sexual maturity. Usually, the normal length of puberty is about 5–6 years. Puberty usually begins later in males than in females, between 9.5 and 13.5 years old (on average 11.5 years old), and the first sign is the testes volume increase (>4 mL), followed by the pubarche within 6 months. After 12–18 months, the enlargement of penis is usually observed. All the clinical modifications that occur during the puberty are the consequence of the hypothalamus–pituitary–gonad axis activation, represented by the increase of the GnRH pulsatility and therefore of FSH, LH, and gonad steroids [49, 50]. The literature reports several studies investigating the relationship between potential endocrine-disrupting agents and the onset of puberty in boys and girls. Many cross-sectional and longitudinal human studies have evaluated the association between pubertal timing onset and prenatal or pubertal exposure to several chemical agents with plausible endocrine interference. Most studies were referred to girls, while few were committed for males’ puberty. Den Hond et al. [51] evaluated 80 boys who were exposed, during the pubertal period, to PCBs and dioxin. They found a negative association between the elevated serum PCBs exposure and pubertal stages, particularly the genital maturation and the pubic hair presentation. On the contrary, they did not find negative effects of dioxin. Saiyed et al. [52] reported an association between pubertal exposure to the pesticide endosulfan and low level of pubic hair, testis, and penis maturation, suggesting a delay in sexual maturation. A work published in 2008 involved 18 girls and 15 boys who were exposed to dioxin-contaminated breast milk. The authors observed a delayed breast development in females and a delayed age at first ejaculation in males [53]. Considering more recent works, Ferguson et al. longitudinally analyzed prenatal and infantile effects of phthalates and BPA on 118 boys (aged 8–14). Prenatal exposure was negatively associated to the adrenarche and pubarche onset (with high SHBG levels), whereas the infantile exposure caused low testosterone levels but no association with puberty was reported [54]. A similar work evaluated the impact of in utero phthalate and BPA exposure on the sexual pubertal maturation of 109 males. In particular, first- and second-trimester pregnancy exposure to DEHP was linked to increased peripubertal serum estradiol levels, whereas third-trimester exposure was associated to a delay on the onset of pubarche, with increased SHBG levels [55]. A longitudinal study conducted on 516 boys considered the effects of many organochlorine chemicals, lead (Pb) and non-dioxin-like PCBs. The authors evaluated EDs concentrations at the age of 8–9 years, and successively annual visits were carried out until the age of 18–19. The endure of blood EDs negatively influenced the growth during the puberty; in particular, dioxin-like compounds, organochlorine pesticides and the Pb delay puberty onset, whereas non-dioxin-like PCBs tend to advance puberty beginning [56]. These studies are summarized in Table 2.
Effects of EDs on anogenital distance
The AGD refers to the distance between the anus and the external genitalia and it is approximately twice the length in male compared to female newborns [57]. AGD is considered a broad biomarker capable of both retrospectively determine early life androgen disruption and predict late-life reproductive disorders in male offspring [57,58,59]. Prenatal androgen action determines reproductive organ size and AGD, and the action can be disrupted by EDs [60,61,62]. Further studies have identified a fetal “masculinization programming window” (MPW), a period from the second to the third month after conception when androgen action could be remarkably affected. The difference of AGD between male and female may be explained by divergent androgen secretions in this period [63]. This sexual dimorphism is apparent in rodents as well as humans [64, 65]. Reproductive parameters at birth and in adulthood appear to be influenced by androgen action in the MPW, similar to their correlation with AGD in human studies [58]. Therefore, a short male AGD is considered a marker of disrupted androgen action. In rodents, a short male AGD largely predicts adverse effect outcomes and it had been used for decades as a marker of impaired fetal androgen action [57].
Animal studies
Compounds most frequently reported to affect male AGD are phthalate esters. Many have been tested in rats, with DBP and DEHP being the most prevalent. Fetal exposure to certain phthalates results in a short AGD in rat male offspring, without any significant effect on female AGD. It is, for the most part, a dose-dependent effect, where increasing dose levels result in progressively shorter AGD, as recently reported [66]. In mice, newborn males exposed to phthalates during the postnatal phase showed a significant short-term reduction of AGD as a possible result of prepubertal hormonal interference [67], although the exact mechanism is still unknown, given the embryonal determination of AGD.
Other substances with a clear antiandrogenic action can affect AGD in rat offspring. Prenatal exposure to high doses of certain AR antagonists, such as pesticide procymidone, vinclozolin, dichlorodiphenyltrichloroethane (DDT), and the non-steroidal prostate cancer drug flutamide, reduces male pup AGD up to 50% compared to controls [68,69,70,71,72]. After exposure to these compounds, the male offspring also displays an increased rate of nipple retention, genital malformations, and severely reduced male reproductive organ weights [57, 68, 73,74,75]. Fetal exposure to both the antimicrobial preservative butyl paraben [76, 77] and the industrial plasticizer BPA [78] has been shown to shorten male AGD around 7–16% in the male offspring, albeit there are studies reporting no effects on AGD for both butyl paraben [79] and bisphenol A [80,81,82,83,84]. PCB exposure in female rats during lactation resulted in reduced AGD in male progeny aged 60 days, even at the lowest dose tested, possibly through a reduction of circulating androgens [85]. Therefore, all these studies suggest that exogenous chemicals can affect AGD in pre- and peri-natally exposed animals.
Human studies
Several epidemiological studies have investigated the effects of EDs on AGD, but the results have been controversial. Most studies focused on male infants, whereas less evidence is available to support a reduction in AGD following prenatal exposure to EDs even in puberty and transition periods. In fact, fetal exposure to different EDs has been frequently associated with a short AGD in newborn boys, in particular phthalates [86,87,88,89,90], but also PFAS [91], dioxins [92], BPA [93, 94], and DDT [95]. Notably, several studies have not found significant correlations between exposure levels and short AGD in boys, including some phthalates [96, 97], DDT [98], triclosan [99], PFAS [100], and various pesticides [101]. These discrepancies do not necessarily diminish the cause for concern, but rather highlight the challenges of obtaining evidence for causal relationships from human epidemiological studies [57]. Swan et al. [89] examined AGD and other genital measurements in relation to prenatal phthalate exposure in 134 newborns aged 2–36 months. The results showed that urinary concentrations of four phthalate metabolites and the summary score were inversely related to AGD. The conclusion supported the hypothesis that prenatal phthalate exposure at environmental levels may adversely affect male reproductive development in humans [89]. Successively, the same group confirmed these results in 2015 [90]; moreover, they observed the same association with reduced AGD when the daily exposures were substantially lower than current US Environmental Protection Agency (EPA) reference doses [102]. Suzuki et al. [88] examined the relationship between prenatal exposure to seven urinary phthalate ester metabolites and AGD in 111 newborns. The results showed the MEHP was negatively associated with AGD. In a Swedish cohort of 196 infants aged 21 months, Bornehag et al. [86] reported a reduced AGD in relation to phthalates concentration in mothers. In a more recent study, the authors investigated phthalates and BPA prenatal exposure in 198 male infants aged 6 months. Surprisingly, the results showed that both MBP and the molar sum of low molecular weight phthalates were positively associated with AGD, although no mechanism to explain this association was suggested [103].
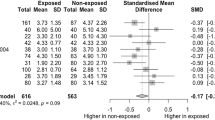
Regarding puberty age, a study on 153 male children aged 0–17 years examined the effect of maternal and paternal exposure to BPA on AGD. Although the cohort included mainly children <5 years old, after correction for age the negative association between either paternal or maternal BPA exposure and AGD remained significant, although the effect was greater when considering mothers’ BPA levels [94]. Another study on young men aged 18–23 reported a reduced AGD associated with maternal exposure to pesticides during pregnancy [104], suggesting that the previously reported associations between EDs prenatal exposure and neonatal and perinatal AGD in male infants can be extended also to later ages. In the same way, these results were also recently confirmed for PFAS: in 212 young men aged 18–19 years, direct exposure to these chemicals resulted in a 10% reduction of AGD [91]. Although PFAS were measured directly in subjects, it could be considered a proxy of prenatal exposure, given the very long half-lives of PFAS [105] and the long-lasting pollution in the geographic area of the exposed subjects. Interestingly, the same study reported a significant negative association between PFAS exposure and another androgen-dependent parameter, penile length [91]. Human studies characteristics are summarized in Table 3.
Effects of EDs on penile length
Penile length is a parameter that positively correlates with postnatal androgen levels [74]. Several studies investigated this correlation, though in newborns. To date, there is only one study that evaluated penile length in pubertal boys in association with EDs exposure: in a cohort of 55 boys aged 11–14, maternal exposure to PCBs during pregnancy was associated with reduced penile length [106]. As previously mentioned, Di Nisio et al. observed similar results in young men aged 18–19, suggesting exposure to EDs can result also in reduced penis size in adolescence [91]. On the contrary, Leijs et al. reported no correlation in 15 young boys (14–19 years of age), exposed to PCBs and dioxin-like compounds, but results were not shown [53].
Effects of EDs on testicular volume
Only few studies investigated the association between EDs and TV in pubertal or transition age. In most studies, TV has been evaluated only for staging puberty [54,55,56]. In addition, studies are extremely heterogeneous, investigating different EDs, considering different primary outcomes, and using different methods for testicular sizing. In fact, TV was evaluated using Prader orchidometer [51, 53, 107,108,109], ultrasound [91], both [110,111,112], or a digital caliper [104]. Moreover, TV may be affected by both in utero and adult exposure [113, 114], with no study investigating the one and the other at the same time. As said before, TV > 4 mL is considered the first pubertal sign, while a TV > 12 mL is considered normal at the end of puberty. Therefore, TV comparison was performed after adjustment for genital stage and in most studies was made according to tertiles or quartiles of exposure, without a control group. Mol et al. [110] observed no significant differences in mean TV, hormonal concentration, and Tanner stage after prenatal PCB exposure in a cohort of children examined at mid puberty. Grandjean et al. [112] evaluated from the same birth cohort 438 adolescent boys, at age 14 years, confirming the same results. On the contrary, Den Hond et al. evaluated postnatal PCB and dioxin-like compounds exposure in 80 boys coming from three different areas of Belgium. The authors reported differences in TV between the areas, even after adjustment for genital stage. However, TV did not correlate with any of the investigated biomarkers of exposure, suggesting it may be a consequence of maternal exposure or other not explored factors [51]. Leijs et al. reported the same no association, but results were not shown [53]. Different results were observed by Cremonese et al., though they used a questionnaire to investigate occupational exposure to pesticides in young Brazilian men. Testicles were larger in rural men, in men using pesticides for more than 1 year, and also among men born to women who worked in agriculture during pregnancy [104]. This is the only study reporting an increase in TV. Authors suggested two possible mechanisms: fetal life exposure with prevalent androgen effect or adult exposure with inflammation of the testicles. Concerning other EDs, Vested et al. [107] recruited 169 young male, aged 19–21 years, whose mothers’ blood had been previously collected during pregnancy. No correlation between PFOA, PFOS, and self-measured TV was observed [107]. Same results were recorded by Joensen et al., who investigated PFAS postnatal exposure in 247 randomly selected healthy young Danish men [111]. On the contrary, Di Nisio et al. reported smaller mean TV in exposed PFAS group (mean age 18.5 ± 0.8 years). Interestingly, both serum and semen PFOA, but not PFOS, were associated with reduced TV [91]. Regarding phthalates, Axelsson et al. investigated prenatal exposure in 112 young Swedish men, aged 17–20 years. Maternal blood samples were recovered in a biobank. The only significant result was that men in the highest tertile of prenatal mono(carboxy-isooctyl) phthalate exposure had smaller TV than men in the lowest [108]. On the contrary, Durmaz et al. found plasma levels of DEHP and MEHP significantly higher in 40 newly diagnosed pubertal gynecomastia cases, aged 11–15 years. However, there was no significant association between their concentration and TV [109]. Studies characteristics are summarized in Table 4.
Effects of EDs on testicular cancer
Few studies investigated a possible correlation between EDs exposure and TC. None of these was performed specifically in adolescents or in transition age. Some papers did not report the age of the studied group or all included patients were older than transition age [115,116,117,118,119]. However, TC is the most common tumor diagnosed in men aged 14–44 years [120]. Most studies evaluated a possible correlation between TC and PCBs. Indeed, the International Agency for Research on Cancer (IARC) rendered PCBs as definite carcinogens in humans [121], while according to the US EPA PCBs cause cancer in animals and are probable human carcinogens (http://www.epa.gov/epawaste/hazard/tsd/pcbs/pubs/effects.htm). However, results regarding TC, as well as other neoplasms, are limited and often discordant. The only study taking into consideration a subgroup of adolescents was performed by Koifman et al. An epidemiological study was carried out by comparing the total amount of pesticides sales in 1985 and health data traced in a National database. A non-statistically significant correlation was observed for TC hospitalization in both age groups (0–14, 15–49 years old) [122]. However, authors did not quantify either the exact amount of pesticides used or the exact area where they were used. In a similar registry-based study, Le Cornet et al. showed no evidence of an association between parental exposure to pesticides and TC [123]. In a cohort of US soldiers, McGlynn et al. reported an increased TC risk in patients with higher plasma levels of DDE and chlordane components [124]. Surprisingly, postnatal PCBs exposure was associated with decreased TC risk in the same cohort [125]. At the same way, Biggs et al. evaluated 246 TC patients after the first course of cancer treatment; however, only 33 were aged 18–24 years (13,4%). TC risk was similar across groups and no trend with increasing serum pesticide levels was observed [126]. Different results were reported by Paoli et al., who observed a statistically significant increase in TC risk in cases with detectable values of total PCB [127]. On the contrary, Hardell et al. investigated both prenatal and postnatal PCB exposure. In patients, only the concentration of cis-nonachlordane was significantly increased, whereas their mothers showed significantly increased concentrations of the sum of PCBs, hexachlorobenzene, trans- and cis-nonachlordane, and the sum of chlordanes [128, 129]. Studies characteristics are summarized in Table 5.
Limitations
Several studies have focused on the effects of EDs, especially on testis development; however, they used different animal models, different EDs doses, and different methodologies, with results not easily comparable among them. In humans, there are only few original papers on the effects of EDs on puberty in males, as the majority focus on female puberty. This aspect could find an explanation in the fact that females’ puberty is more easily detectable, since menarche represents an undisputed sign of sexual maturation. Given the lack of homogeneity across the different studies, because of the wide spectrum of EDs and different exposure sources, age of subjects, and analytical measurements, it is difficult to infer a comprehensive conclusion on the effect of EDs on puberty and transition period. Moreover, we should consider the lack of longitudinal studies in the literature, and in most cases the quantification of EDs levels was performed only in mothers during pregnancy or only in children after birth, thus not providing a direct assessment of mother–fetus exposure levels. In other cases, the analytical measurement of chemicals is missing and it is only presumed by occupational exposure. In addition, humans are exposed to several chemicals, which could affect male health in a dose-additive manner. In fact, testicular toxicity was reported in rats exposed to a mixture of phthalates, though dosage of each was below the adverse effect threshold [130]. Therefore, bioaccumulation in adipose tissue of several EDs may be responsible of a “cocktail effect,” a possible sum of effects with unknown outcomes that may unexpectedly appear after years of exposure to low dosages [131]. Moreover, as said before, recent data suggest that EDs detrimental effects may even be inherited by future generations [132]. All these factors may partially contribute to the heterogeneity of results reported in literature. In the same way, lifestyle factors in young adults should not be neglected. An increasing trend of health risk behaviors, such as smoking, use of illegal drugs and alcohol, has been recently reported in adolescents. All these behaviors have been associated with andrological disorders and could impair testicular development [133].
Conclusions
Current evidence does not clarify the impact of EDs on human male reproductive health. In animal models, severe harmful effects were observed. However, human studies have shown controversial results. This discrepancy may be due to several factors, as said above. Despite the lack of consistency in the results, overall conclusion points toward a positive association between exposure to EDs and reproductive system damage. Among the studies based on the consequences of EDs exposure on males’ puberty, the main findings concern on delayed puberty, probably associated to the xeno-estrogens effects of PCBs, polychlorinated dibenzofurans, and endosulfan. In the same way, by using AGD as a proxy of fetal exposure to endocrine-disrupting chemicals, most studies agree on an antiandrogenic effect of different classes of EDs (phthalates, BPA, pesticides, PFAS), which results in reduced AGD after birth, as measured from newborns until transition period. On the contrary, the correlation between EDs and TV, as well as that with testicular cancer, is more uncertain. EDs mechanisms of damage and the adverse consequences on male puberty and andrological health are summarized in Fig. 1. Although the observed effects may be subtle on an individual level, the biological link between them (i.e., TDS: decreased androgen levels contributing to cryptorchidism, reduced penile length, reduced TV) should raise concern about the effects of EDs at population levels in young men. Further long-term studies performed on a wide number of subjects are necessary in order to identify damaging compounds, clarify sources of exposure, and replace them with harmless substances.
Change history
04 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12020-020-02581-1
References
R. Hauser, N.E. Skakkebaek, U. Hass et al., Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 100(4), 1267–1277 (2015). https://doi.org/10.1210/jc.2014-4325
S. Sifakis, V.P. Androutsopoulos, A.M. Tsatsakis, D.A. Spandidos, Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 51, 56–70 (2017). https://doi.org/10.1016/j.etap.2017.02.024
A. Soubry, J.M. Schildkraut, A. Murtha et al., Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Med. 11, 29 (2013). https://doi.org/10.1186/1741-7015-11-29
A. Soubry, S.K. Murphy, F. Wang et al., Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 39(4), 650–657 (2015). https://doi.org/10.1038/ijo.2013.193
I. Katsikantami, S. Sifakis, M.N. Tzatzarakis et al., A global assessment of phthalates burden and related links to health effects. Environ. Int. 97, 212–236 (2016). https://doi.org/10.1016/j.envint.2016.09.013
M.F. Sweeney, N. Hasan, A.M. Soto, C. Sonnenschein, Environmental endocrine disruptors: effects on the human male reproductive system. Rev. Endocr. Metab. Disord. 16(4), 341–357 (2015). https://doi.org/10.1007/s11154-016-9337-4
N.E. Skakkebaek, E. Rajpert-De Meyts, G.M. Buck Louis et al., Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96(1), 55–97 (2016). https://doi.org/10.1152/physrev.00017.2015
M.H. Hsieh, B.N. Breyer, M.L. Eisenberg, L.S. Baskin, Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr. Urol. Rep. 9(2), 137–142 (2008). https://doi.org/10.1007/s11934-008-0025-0
M.H. Hsieh, M.L. Eisenberg, A.B. Hittelman, J.M. Wilson, G.E. Tasian, L.S. Baskin, Caucasian male infants and boys with hypospadias exhibit reduced anogenital distance. Hum. Reprod. 27(6), 1577–1580 (2012). https://doi.org/10.1093/humrep/des087
V.G. Jain, A.K. Singal, Shorter anogenital distance correlates with undescended testis: a detailed genital anthropometric analysis in human newborns. Hum. Reprod. 28(9), 2343–2349 (2013). https://doi.org/10.1093/humrep/det286
A. Thankamony et al., Anogenital distance and penile length in infants with hypospadias or cryptorchidism: comparison with normative data. Environ. Health Perspect. 122(2), 207–211 (2014)
M.L. Eisenberg, M.H. Hsieh, R.C. Walters, R. Krasnow, L.I. Lipshultz, The relationship between anogenital distance, fatherhood, and fertility in adult men. PLoS ONE 6(5), e18973 (2011). https://doi.org/10.1371/journal.pone.0018973.
M.L. Eisenberg, L.I. Lipshultz, Anogenital distance as a measure of human male fertility. J. Assist. Reprod. Genet. 32(3), 479–484 (2015). https://doi.org/10.1007/s10815-014-0410-1
J. Mendiola, M. Melgarejo, M. Moñino-García, A. Cutillas-Tolín, J.A. Noguera-Velasco, A.M. Torres-Cantero, Is anogenital distance associated with semen quality in male partners of subfertile couples? Andrology 3(4), 672–676 (2015). https://doi.org/10.1111/andr.12059
J.J. Arnett, Emerging adulthood. A theory of development from the late teens through the twenties. Am. Psychol. 55(5), 469–480 (2000)
A. Colver, S. Longwell, New understanding of adolescent brain development: relevance to transitional healthcare for young people with long term conditions. Arch. Dis. Child. 98(11), 902–907 (2013)
K.R. Kilcoyne, R.T. Mitchell, Effect of environmental and pharmaceutical exposures on fetal testis development and function: a systematic review of human experimental data. Hum. Reprod. Update 25(4), 397–421 (2019)
V. Rouiller-Fabre, R. Habert, G. Livera, Effects of endocrine disruptors on the human fetal testis. Ann. Endocrinol. 75, 54–57 (2014)
B. Lu, C.E. Bishop, Late onset of spermatogenesis and gain of fertility inPOG-deficient mice indicate that POG is not necessary for the proliferation of spermatogonia. Biol. Reprod. 69, 161–168 (2003)
V. Rouiller-Fabre, R. Lambrot, V. Muczynski, H. Coffigny, C. Lécureuil, C. Pairault et al., Development and regulations of testicular functions in the human foetus. Gynecol. Obstet. Fertil. 36(9), 898–907 (2008)
A.M. Heeren, L. van Iperen, D.B. Klootwijk, A. de Melo Bernardo, M.S. Roost, M.M. Gomes Fernandes, L.A. Louwe, C.G. Hilders, F.M. Helmerhorst, L.A. van der Westerlaken et al., Development of the follicular basement membrane during human gam-etogenesis and early folliculogenesis. BMC Dev. Biol. 15, 4 (2015)
M.J. Bitgood, L. Shen, A.P. McMahon, Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 6, 298–304 (1996)
S.L. Griswold, R.R. Behringer, Fetal Leydig cell origin and development. Sex Dev. 3, 1–15 (2009)
I.A. Hughes, C.L. Acerini, Factors controlling testis descent. Eur. J. Endocrinol. 159, S75–S82 (2008)
S. van den Driesche, K.R. Kilcoyne, I. Wagner, D. Rebourcet, A. Boyle, R. Mitchell, C. McKinnell, S. Macpherson, R. Donat, C.J. Shukla et al., Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2, e91204 (2017)
R. Habert, G. Livera, V. Rouiller-Fabre, Man is not a big rat: concerns with traditional human risk assessment of phthalates based on their anti-androgenic effects observed in the rat foetus. Basic Clin. Androl. 24, 14 (2014)
A. Lehraiki, C. Racine, A. Krust, R. Habert, C. Levacher, Phthalates impair germ cell number in the mouse fetal testis by an androgen- and estrogen-independent mechanism. Toxicol. Sci. 111(2), 372–382 (2009)
K.W. Gaido, J.B. Hensley, D. Liu, D.G. Wallace, S. Borghoff, K.J. Johnson, S.J. Hall, K. Boekelheide, Fetal mouse phthalate exposure shows that gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 97(2), 491–503 (2007)
N.E. Heger, S.J. Hall, M.A. Sandrof, E.V. McDonnell, J.B. Hensley, E.N. McDowell et al., Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ. Health Perspect. 120(8), 1137–1143 (2012)
R. Lambrot, V. Muczynski, C. Lécureuil, G. Angenard, H. Coffigny, C. Pairault et al., Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ. Health Perspect. 117, 32–37 (2009)
R.T. Mitchell, A.J. Childs, R.A. Anderson, S. van den Driesche, P.T. Saunders, C. McKinnell et al., Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate. J. Clin. Endocrinol. Metab. 97(3), E341–E348 (2012)
O. Albert, B. Jegou, A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum. Reprod. Update 20, 231–249 (2014)
D.J. Spade, S.J. Hall, S. Wilson, K. Boekelheide, Di-n-Butyl phthalate induces multinucleated germ cells in the rat fetal testis through a nonproliferative mechanism. Biol. Reprod. 93(5), 110 (2015). https://doi.org/10.1095/biolreprod.115.131615
S. Eladak, D. Moison, M.J. Guerquin, G. Matilionyte, K. Kilcoyne, T. N’Tumba-Byn, S. Messiaen, Y. Deceuninck, S. Pozzi-Gaudin, A. Benachi et al., Effects of environmental bisphenol A exposures on germ cell development and Leydig cell function in the human fetal testis. PLoS ONE 13, e0191934 (2018)
T. N’Tumba-Byn, D. Moison, M. Lacroix, C. Lecureuil, L. Lesage, S.M. Prud’homme et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal Leydig cell function. PLoS ONE 7(12), e51579 (2012)
M.D. Komarowska, A. Hermanowicz, U. Czyzewska, R. Milewski, E. Matuszczak, W. Miltyk, W. Debek, Serum bisphenol A level in boys with cryptorchidism: a step to male infertility? Int. J. Endocrinol. 2015, 973154 (2015)
C. Chevrier, C. Petit, C. Philippat, M. Mortamais, R. Slama, F. Rouget, A.M. Calafat, X. Ye, M.J. Silva, M.A. Charles et al., Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology 23, 353–356 (2012)
J.G. Teeguarden, N.C. Twaddle, M.I. Churchwell, D.R. Doerge, Urine and serum biomonitoring of exposure to environmental estrogens I: bisphenol A in pregnant women. Food Chem. Toxicol. 92, 129–142 (2016)
J. Toppari, J.C. Larsen, P. Christiansen, A. Giwercman, P. Grandjean, L.J. Guillette Jr et al., Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 104(Suppl. 4), 741–803 (1996)
J. Toppari, Environmental endocrine disrupters. Sex Dev. 2, 260–267 (2008)
E. Sugantha Priya, T. Sathish Kumar, S. Balaji, S. Bavithra, P. Raja Singh, D. Sakthivel, B. Ravi Sankar, J. Arunakaran, Lactational exposure effect of polychlorinated biphenyl on rat Sertoli cell markers and functional regulators in prepuberal and puberal F1 offspring. J. Endocrinol. Investig. 40(1), 91–100 (2017)
A. Eggert, S. Cisneros-Montalvo, S. Anandan, S. Musilli, J.B. Stukenborg, A. Adamsson, M. Nurmio, J. Toppari, The effects of perfluorooctanoic acid (PFOA) on fetal and adult rat testis. Reprod. Toxicol. 90, 68–76 (2019)
H. Lu, H. Zhang, J. Gao, Z. Li, S. Bao, X. Chen, Y. Wang, R. Ge, L. Ye, Effects of perfluorooctanoic acid on stem Leydig cell functions in the rat. Environ. Pollut. 250, 206–215 (2019)
B. Mao, D. Mruk, Q. Lian, R. Ge, C. Li, B. Silvestrini, C.Y. Cheng, Mechanistic insights into PFOS-mediated sertoli cell injury. Trends Mol. Med. 24(9), 781–793 (2018)
Y. Gao, H. Chen, X. Xiao, W.Y. Lui, W.M. Lee, D.D. Mruk, C.Y. Cheng, Perfluorooctanesulfonate (PFOS)-induced Sertoli cell injury through a disruption of F-actin and microtubule organization is mediated by Akt1/2. Sci. Rep. 7(1), 1110 (2017). https://doi.org/10.1038/s41598-017-01016-8
L. Li, X. Li, X. Chen et al., Perfluorooctane sulfonate impairs rat Leydig cell development during puberty. Chemosphere 190, 43–53 (2018)
G. Toft, B.A. Jönsson, J.P. Bonde, B. Nørgaard-Pedersen, D.M. Hougaard, A. Cohen, C.H. Lindh, R. Ivell, R. Anand-Ivell, M.S. Lindhard, Perfluorooctane sulfonate concentrations in amniotic fluid, biomarkers of fetal leydig cell function, and cryptorchidism and hypospadias in danish boys (1980-1996). Environ. Health Perspect. 124(1), 151–156 (2016)
A. Sansone, S. Kliesch, A.M. Isidori, S.A.M.H. Schlatt, and INSL3 in testicular and extragonadal pathophysiology: what do we know? Andrology 7(2), 131–138 (2019). https://doi.org/10.1111/andr.12597
W.A. Marshall, J.M. Tanner, Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 44, 291–303 (1969)
W.A. Marshall, J.M. Tanner, Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 45, 13–23 (1970)
E. Den Hond, H.A. Roels, K. Hoppenbrouwers et al., Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ. Health Perspect. 110(8), 771–776 (2002)
H. Saiyed, A. Dewan, V. Bhatnagar et al., Effect of endosulfan on male reproductive development. Environ. Health Perspect. 111(16), 1958–1962 (2003). https://doi.org/10.1289/ehp.6271
M.M. Leijs, J.G. Koppe, K. Olie, W.M. van Aalderen, P. Voogt, T. Vulsma, M. Westra, G.W. ten Tusscher, Delayed initiation of breast development in girls with higher prenatal dioxin exposure; a longitudinal cohort study. Chemosphere 73, 999–1004 (2008)
K.K. Ferguson, K.E. Peterson, J.M. Lee, A. Mercado-García, C.B. Goldenberg, M.M. Téllez-Rojo, J.D. Meeker, Prenatal and peripubertal phthalates and bisphenol-A in relation to sex hormones and puberty in boys. Reprod. Toxicol. 47, 70–76 (2014).
D.J. Watkins, B.N. Sánchez, M.M. Téllez-Rojo et al., Impact of phthalate and BPA exposure during in utero windows of susceptibility on reproductive hormones and sexual maturation in peripubertal males. Environ. Health 16, 69 (2017). https://doi.org/10.1186/s12940-017-0278-5
O. Sergeyev, J.S. Burns, P.L. Williams, S.A. Korrick, M.M. Lee, B. Revich, R. Hauser, The association of peripubertal serum concentrations of organochlorine chemicals and blood lead with growth and pubertal development in a longitudinal cohort of boys: a review of published results from the Russian Children’s Study. Environ. Health. 32(1–2), 83–92 (2017)
C.L. Schwartz, S. Christiansen, A.M. Vinggaard, M. Axelstad, U. Hass, T. Svingen, Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders. Arch. Toxicol. 93(2), 253–272 (2019).
A. Dean, R.M. Sharpe, Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J. Clin. Endocrinol. Metab. 98(6), 2230–2238 (2013)
A. Thankamony, V. Pasterski, K.K. Ong, C.L. Acerini, I.A. Hughes, Anogenital distance as a marker of androgen exposure in humans. Andrology 4(4), 616–625 (2016). https://doi.org/10.1111/andr.12156
C.L. Acerini, I.A. Hughes, Endocrine disrupting chemicals: a new and emerging public health problem? Arch. Dis. Child. 91(8), 633–641 (2006). https://doi.org/10.1136/adc.2005.088500
E. Diamanti-Kandarakis, E. Palioura, S.A. Kandarakis, M. Koutsilieris, The impact of endocrine disruptors on endocrine targets. Horm. Metab. Res. 42(8), 543–552 (2010). https://doi.org/10.1055/s-0030-1252034
J.A. Taylor, C.A. Richter, R.L. Ruhlen, F.S. vom Saal, Estrogenic environmental chemicals and drugs: mechanisms for effects on the developing male urogenital system. J. Steroid Biochem. Mol. Biol. 127(1–2), 83–95 (2011). https://doi.org/10.1016/j.jsbmb.2011.07.005
H.M. Scott, G.R. Hutchison, M.S. Jobling, C. McKinnell, A.J. Drake, R.M. Sharpe, Relationship between androgen action in the “male programming window,” fetal sertoli cell number, and adult testis size in the rat. Endocrinology. 149(10), 5280–5287 (2008). https://doi.org/10.1210/en.2008-0413
E. Salazar-Martinez, P. Romano-Riquer, E. Yanez-Marquez, M.P. Longnecker, M. Hernandez-Avila, Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environ. Health 3(1), 8 (2004). https://doi.org/10.1186/1476-069X-3-8
A. Thankamony, K.K. Ong, D.B. Dunger, C.L. Acerini, I.A. Hughes, Anogenital distance from birth to 2 years: a population study. Environ. Health Perspect. 117(11), 1786–1790 (2009). https://doi.org/10.1289/ehp.0900881
T. Ma, X. Yin, R. Han, J. Ding, H. Zhang, X. Han, D. Li, Effects of In utero exposure to di-n-butyl phthalate on testicular development in rat. Int. J. Environ. Res. Public Health 14(10), E1284 (2017). https://doi.org/10.3390/ijerph14101284
S. Moody, H. Goh, A. Bielanowicz, P. Rippon, K.L. Loveland, C. Itman, Prepubertal mouse testis growth and maturation and androgen production are acutely sensitive to di-n-butyl phthalate. Endocrinology 154(9), 3460–3475 (2013). https://doi.org/10.1210/en.2012-2227
S. Christiansen, M. Scholze, M. Axelstad, J. Boberg, A. Kortenkamp, U. Hass, Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int. J. Androl. 31, 241–248 (2008). https://doi.org/10.1111/j.1365-2605.2008.00866.x
U. Hass, M. Scholze, S. Christiansen et al., Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ. Health Perspect. 115(Suppl. 1), 122–128 (2007). https://doi.org/10.1289/ehp.9360
J. Ostby, W.R. Kelce, C. Lambright, C.J. Wolf, P. Mann, L.E. Gray Jr., The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol. Ind. Health 15(1–2), 80–93 (1999). https://doi.org/10.1177/074823379901500108
L.G. Parks, J.S. Ostby, C.R. Lambright et al., The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 58(2), 339–349 (2000). https://doi.org/10.1093/toxsci/58.2.339
S.M. Patrick, M.S. Bornman, A.M. Joubert, N. Pitts, V. Naidoo, C. de Jager, Effects of environmental endocrine disruptors, including insecticides used for malaria vector control on reproductive parameters of male rats. Reprod. Toxicol. 61, 19–27 (2016). https://doi.org/10.1016/j.reprotox.2016.02.015
C.J. Bowman, N.J. Barlow, K.J. Turner, D.G. Wallace, P.M. Foster, Effects of in utero exposure to finasteride on androgen-dependent reproductive development in the male rat. Toxicol. Sci. 74(2), 393–406 (2003). https://doi.org/10.1093/toxsci/kfg128
M. Welsh, P.T. Saunders, M. Fisken, H.M. Scott, G.R. Hutchison, L.B. Smith, R.M. Sharpe, Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 118(4), 1479–1490 (2008)
M. Welsh, D.J. MacLeod, M. Walker, L.B. Smith, R.M. Sharpe, Critical androgen-sensitive periods of rat penis and clitoris development. Int. J. Androl. 33(1), e144–e152 (2010). https://doi.org/10.1111/j.1365-2605.2009.00978.x
J. Boberg, M. Axelstad, T. Svingen, K. Mandrup, S. Christiansen, A.M. Vinggaard, U. Hass, Multiple endocrine disrupting effects in rats perinatally exposed to butylparaben. Toxicol. Sci. 152(1), 244–256 (2016). https://doi.org/10.1093/toxsci/kfw079
L. Zhang, L. Dong, S. Ding, P. Qiao, C. Wang, M. Zhang, L. Zhang, Q. Du, Y. Li, N. Tang, B. Chang, Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E2 levels in male rat offspring. Environ. Toxicol. Pharmacol. 37(2), 705–717 (2014)
S. Christiansen, M. Axelstad, J. Boberg, A.M. Vinggaard, G.A. Pedersen, U. Hass, Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 147(4), 477–487 (2014). https://doi.org/10.1530/REP-13-0377
C. Taxvig, A.M. Vinggaard, U. Hass, M. Axelstad, J. Boberg, P.R. Hansen et al., Do parabens have the ability to interfere with steroidogenesis? Toxicol. Sci. 106, 206–213 (2008). https://doi.org/10.1093/toxsci/kfn148
E. Spörndly-Nees, J. Boberg, E. Ekstedt, L. Holm, A. Fakhrzadeh, L. Dunder, M.M. Kushnir, M.H. Lejonklou, P.M. Lind, Low-dose exposure to bisphenol A during development has limited effects on male reproduction in midpubertal and aging Fischer 344 rats. Reprod. Toxicol. 81, 196–206 (2018). https://doi.org/10.1016/j.reprotox.2018.08.007
S.A. Ferguson, C.D.J. Law, J.S. Abshire, Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 124(1), 149–160 (2011)
K.L. Howdeshell, J. Furr, C.R. Lambright, V.S. Wilson, B.C. Ryan, L.E. Gray Jr., Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol. Sci. 102(2), 371–382 (2008)
H. Takagi, M. Shibutani, N. Masutomi, C. Uneyama, N. Takahashi, K. Mitsumori, M. Hirose, Lack of maternal dietary exposure effects of bisphenol A and nonylphenol during the critical period for brain sexual differentiation on the reproductive/endocrine systems in later life. Arch. Toxicol. 78(2), 97–105 (2004)
H. Tinwell, J. Haseman, P.A. Lefevre, N. Wallis, J. Ashby, Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol. Sci. 68, 339–348 (2002)
S.K. Thangavelu, S.P. Elaiyapillai, I. Ramachandran, R.S. Bhaskaran, A. Jagadeesan, Lactational exposure of polychlorinated biphenyls impair Leydig cellular steroidogenesis in F(1) progeny rats. Reprod. Toxicol. 75, 73–85 (2018). https://doi.org/10.1016/j.reprotox.2017.11.009
C.G. Bornehag, F. Carlstedt, B.A. Jönsson, C.H. Lindh, T.K. Jensen, A. Bodin, C. Jonsson, S. Janson, S.H. Swan, Prenatal phthalate exposures and anogenital distance in Swedish boys. Environ. Health Perspect. 123(1), 101–107 (2015). https://doi.org/10.1289/ehp.1408163
L.P. Bustamante-Montes, M.A. Hernández-Valero, D. Flores-Pimentel et al., Prenatal exposure to phthalates is associated with decreased anogenital distance and penile size in male newborns. J. Dev. Orig. Health Dis. 4(4), 300–306 (2013). https://doi.org/10.1017/S2040174413000172
Y. Suzuki, J. Yoshinaga, Y. Mizumoto, S. Serizawa, H. Shiraishi, Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 35(3), 236–244 (2012). https://doi.org/10.1111/j.1365-2605.2011.01190.x
S.H. Swan, K.M. Main, F. Liu, S.L. Stewart, R.L. Kruse, A.M. Calafat, C.S. Mao, J.B. Redmon, C.L. Ternand, S. Sullivan, J.L. Teague, Study for Future Families Research Team, Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ. Health Perspect. 113(8), 1056–1061 (2005).
S.H. Swan, S. Sathyanarayana, E.S. Barrett et al., First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod. 30(4), 963–972 (2015). https://doi.org/10.1093/humrep/deu363
A. Di Nisio, I. Sabovic, U. Valente, S. Tescari, M.S. Rocca, D. Guidolin, S. Dall’Acqua, L. Acquasaliente, N. Pozzi, M. Plebani, A. Garolla, C. Foresta, Endocrine disruption of androgenic activity by perfluoroalkyl substances: clinical and experimental evidence. J. Clin. Endocrinol. Metab. 2018. https://doi.org/10.1210/jc.2018-01855
M. Vafeiadi, S. Agramunt, E. Papadopoulou, H. Besselink, K. Mathianaki, P. Karakosta, A. Spanaki, A. Koutis, L. Chatzi, M. Vrijheid, M. Kogevinas, In utero exposure to dioxins and dioxin-like compounds and anogenital distance in newborns and infants. Environ. Health Perspect. 121(1), 125–130 (2013). https://doi.org/10.1289/ehp.1205221
E. Mammadov, M. Uncu, C. Dalkan, High prenatal exposure to bisphenol A reduces anogenital distance in healthy male newborns. J. Clin. Res. Pediatr. Endocrinol. 10(1), 25–29 (2018). https://doi.org/10.4274/jcrpe.4817
M. Miao, W. Yuan, Y. He, Z. Zhou, J. Wang, E. Gao, G. Li, D.K. Li, In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. A Clin. Mol. Teratol. 91(10), 867–872 (2011). https://doi.org/10.1002/bdra.22845
L. Torres-Sanchez, M. Zepeda, M.E. Cebrián, J. Belkind-Gerson, R.M. Garcia-Hernandez, U. Belkind-Valdovinos, L. López-Carrillo, Dichlorodiphenyldichloroethylene exposure during the first trimester of pregnancy alters the anal position in male infants. Ann. N. Y. Acad. Sci. 1140, 155–162 (2008). https://doi.org/10.1196/annals.1454.004
T.K. Jensen, H. Frederiksen, H.B. Kyhl et al., Prenatal exposure to phthalates and anogenital distance in male infants from a low-exposed Danish cohort (2010-2012). Environ. Health Perspect. 124(7), 1107–1113 (2016). https://doi.org/10.1289/ehp.1509870
P.C. Huang, P.L. Kuo, Y.Y. Chou, S.J. Lin, C.C. Lee, Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 35(1), 14–20 (2009)
M.S. Bornman, J. Chevrier, S. Rauch et al., Dichlorodiphenyltrichloroethane exposure and anogenital distance in the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) birth cohort study, South Africa. Andrology 4(4), 608–615 (2016). https://doi.org/10.1111/andr.12235
T.H. Lassen, H. Frederiksen, H.B. Kyhl, S.H. Swan, K.M. Main, A.M. Andersson, D.V. Lind, S. Husby, C. Wohlfahrt-Veje, N.E. Skakkebæk, T.K. Jensen, Prenatal triclosan exposure and anthropometric measures including anogenital distance in Danish infants. Environ. Health Perspect. 124(8), 1261–1268 (2016). https://doi.org/10.1289/ehp.1409637
D.V. Lind, L. Priskorn, T.H. Lassen, F. Nielsen, H.B. Kyhl, D.M. Kristensen, H.T. Christesen, J.S. Jorgensen, P. Grandjean, T.K. Jensen, Prenatal exposure to perfluoroalkyl substances and anogenital distance at 3 months of age in a Danish mother-child cohort. Reprod. Toxicol. 68, 200–206 (2017). https://doi.org/10.1016/j.reprotox.2016.08.019
L. Dalsager, L.E. Christensen, M.G. Kongsholm et al., Associations of maternal exposure to organophosphate and pyrethroid insecticides and the herbicide 2,4-D with birth outcomes and anogenital distance at 3 months in the Odense child cohort. Reprod. Toxicol. 76, 53–62 (2018). https://doi.org/10.1016/j.reprotox.2017.12.008
K. Marsee, T.J. Woodruff, D.A. Axelrad, A.M. Calafat, S.H. Swan, Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance [published correction appears in Environ Health Perspect. 2007 Feb;115(2):A73]. Environ. Health Perspect. 114(6), 805–809 (2006). https://doi.org/10.1289/ehp.8663
T.E. Arbuckle, A. Agarwal, S.H. MacPherson, W.D. Fraser, S. Sathyanarayana, T. Ramsay, L. Dodds, G. Muckle, M. Fisher, W. Foster, M. Walker, P. Monnier, Prenatal exposure to phthalates and phenols and infant endocrine-sensitive outcomes: The MIREC study. Environ. Int. 120, 572–583 (2018). https://doi.org/10.1016/j.envint.2018.08.034
C. Cremonese, C. Piccoli, F. Pasqualotto, R. Clapauch, R.J. Koifman, S. Koifman, C. Freire, Occupational exposure to pesticides, reproductive hormone levels and sperm quality in young Brazilian men. Reprod. Toxicol. 67, 174–185 (2017). https://doi.org/10.1016/j.reprotox.2017.01.001
J. Stubleski, S. Salihovic, P.M. Lind, L. Lind, L. Dunder, P. McCleaf et al., The effect of drinking water contaminated with perfluoroalkyl substances on a 10-year longitudinal trend of plasma levels in an elderly uppsala cohort. Environ. Res. 159, 95–10 (2017)
Y.L. Guo, G.H. Lambert, C.C. Hsu et al., Yucheng: health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. Int. Arch. Occupat. Environ. Health 77(3), 153–158 (2004)
A. Vested, C.H. Ramlau-Hansen, S.F. Olsen et al., Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Perspect. 121(4), 453–458 (2013). https://doi.org/10.1289/ehp.1205118
J. Axelsson, L. Rylander, A. Rignell-Hydbom, C.H. Lindh, B.A. Jonsson, A. Giwercman, Prenatal phthalate exposure and reproductive function in young men. Environ. Res. 138C, 264–270 (2015)
E. Durmaz, E.N. Ozmert, P. Erkekoglu, B. Giray, O. Derman, F. Hincal, K. Yurdakök, Plasma phthalate levels in pubertal gynecomastia. Pediatrics 125, 122–129 (2010)
N.M. Mol, N. Sorensen, P. Weihe et al., Spermaturia and serum hormone concentrations at the age of puberty in boys prenatally exposed to polychlorinated biphenyls. Eur. J. Endocrinol. 146(3), 357–363 (2002)
U.N. Joensen, B. Veyrand, J.P. Antignac, M. Blomberg Jensen, J.H. Petersen, P. Marchand, et al., PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum. Reprod. 28(3), 599–608 (2013). https://doi.org/10.1093/humrep/des425
P. Grandjean, C. Grønlund, I.M. Kjær et al., Reproductive hormone profile and pubertal development in 14-year-old boys prenatally exposed to polychlorinated biphenyls. Reprod. Toxicol. 34(4), 498–503 (2012). https://doi.org/10.1016/j.reprotox.2012.07.005
M. Nakai et al., Acute and long-term effects of a single dose of the fungicide carbendazim (Methyl 2-Benzimidazole Carbamate) on the male reproductive system in the rat. J. Androl. 13, 507–518 (1992)
A. Cardone, R. Comitato, F. Angelini, Spermatogenesis, epididymis morphology and plasma sex steroid secretion in the male lizard Podarcis sicula exposed to diuron. Environ, Res. 108, 214–223 (2008)
V. Barry, A. Winquist, K. Steenland, Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 121(11–12), 1313–1318 (2013). https://doi.org/10.1289/ehp.1306615
M. Benedetti, A. Zona, E. Beccaloni, M. Carere, P. Comba, Incidence of breast, prostate, testicular, and thyroid cancer in italian contaminated sites with presence of substances with endocrine disrupting properties. Int. J. Environ. Res. Public Health 14(4), 355 (2017). https://doi.org/10.3390/ijerph14040355
C.G. Ohlson, L. Hardell, Testicular cancer and occupational exposures with a focus on xenoestrogens in polyvinyl chloride plastics. Chemosphere 40, 1277–1282 (2000). https://doi.org/10.1016/S0045-6535(99)00380-X
M.P. Purdue, L.S. Engel, H. Langseth et al., Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ. Health Perspect. 117(10), 1514–1519 (2009). https://doi.org/10.1289/ehp.0800359
V.M. Vieira, K. Hoffman, H.M. Shin, J.M. Weinberg, T.F. Webster, T. Fletcher, Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ. Health Perspect. 121(3), 318–323 (2013). https://doi.org/10.1289/ehp.1205829
L. Cheng, P. Albers, D.M. Berney, D.R. Feldman, G. Daugaard, T. Gilligan et al., Testicular cancer. Nat. Rev. Dis. Primers 4, 29 (2018). https://doi.org/10.1038/s41572-018-0029-0
Monograph IARC Working Group, Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 14, 287–288 (2013)
S. Koifman, R.J. Koifman, A. Meyer, Human reproductive system disturbances and pesticide exposure in Brazil. Cad. Saude Publica 18, 435–445 (2002)
C. Le Cornet, B. Fervers, S.O. Dalton, M. Feychting, E. Pukkala, T. Tynes et al., Testicular germ cell tumours and parental occupational exposure to pesticides: a register-based case-control study in the Nordic countries (NORD-TEST study). Occup. Environ. Med. 72(11), 805–811 (2015). https://doi.org/10.1136/oemed-2015-102860
K.A. McGlynn, S.M. Quraishi, B.I. Graubard, J.P. Weber, M.V. Rubertone, R.L. Erickson, Persistent organochlorine pesticides and risk of testicular germ cell tumors. J. Natl. Cancer Inst. 100, 663–671 (2008)
K.A. McGlynn, S.M. Quraishi, B.I. Graubard, J.P. Weber, M.V. Rubertone, R.L. Erickson, Polychlorinated biphenyls and risk of testicular germ cell tumors. Cancer Res. 69(5), 1901–1909 (2009). https://doi.org/10.1158/0008-5472.CAN-08-3935
M.L. Biggs, M.D. Davis, D.L. Eaton et al., Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: a population-based case-control study. Cancer Epidemiol. Biomarkers Prev. 17(8), 2012–2018 (2008). https://doi.org/10.1158/1055-9965.EPI-08-0032
D. Paoli, F. Giannandrea, M. Gallo, R. Turci, M.S. Cattaruzza, F. Lombardo et al., Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J. Endocrinol. Investig. 38, 745–752 (2015). https://doi.org/10.1007/s40618-015-0251-5
L. Hardell, B. van Bavel, G. Lindström et al., Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ. Health Perspect. 111(7), 930–934 (2003). https://doi.org/10.1289/ehp.5816
L. Hardell, B. Van Bavel, G. Lindstrom, M. Carlberg, M. Eriksson, A.C. Dreifaldt et al., Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int. J. Androl. 27, 282–290 (2004). https://doi.org/10.1111/j.1365-2605.2004.00489.x
A.J. Martino-Andrade, I. Chahoud, Reproductive toxicity of phthalate esters. Mol. Nutr. Food Res. 54(1), 148–157 (2010). https://doi.org/10.1002/mnfr.200800312
F. Pallotti, M. Pelloni, D. Gianfrilli, A. Lenzi, F. Lombardo, D. Paoli, Mechanisms of testicular disruption from exposure to bisphenol A and phtalates. J. Clin. Med. 9(2), 471 (2020). https://doi.org/10.3390/jcm9020471
R. Lauretta, A. Sansone, M. Sansone, F. Romanelli, M. Appetecchia, Endocrine disrupting chemicals: effects on endocrine glands. Front. Endocrinol. 10, 178 (2019). https://doi.org/10.3389/fendo.2019.00178
D. Gianfrilli, A. Ferlin, A.M. Isidori et al., Risk behaviours and alcohol in adolescence are negatively associated with testicular volume: results from the Amico-Andrologo survey. Andrology 7(6), 769–777 (2019). https://doi.org/10.1111/andr.12659
Acknowledgements
This study has been proposed and scientifically supported by the TALENT Study Group, Sapienza University of Rome, Italy. Open access funding provided by Università degli Studi di Roma La Sapienza within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors were involved in the design of this study and made substantial contributions to this paper. All authors critically revised and approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cargnelutti, F., Di Nisio, A., Pallotti, F. et al. Effects of endocrine disruptors on fetal testis development, male puberty, and transition age. Endocrine 72, 358–374 (2021). https://doi.org/10.1007/s12020-020-02436-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02436-9