Abstract
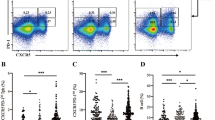
Type 1 diabetes (T1D) is perceived as an autoimmune disease caused by T cell-mediated destruction of the insulin-producing pancreatic β cells. However, the number of inflammatory T cells in blood, as well as the relative importance of each cell type is unclear. Forty-two patients with T1D and 30 controls were enrolled. Circulating primary CD4+ or CD8+ T cells were quantified with 5-color flow cytometry. Serum IL-22 and IL-17 levels were examined by ELISA. Serum autoantibodies were measured by radio-binding assays, using 35S-labeled glutamic acid decarboxylase-65 (GAD65), protein tyrosine phosphatase-2 (IA-2), and zinc transporter 8 (ZnT8). Th17–Th22 and Tc1–Tc17 were significantly elevated in patients with T1D compared to control subjects, while there were no significant differences in Th1 cells. The levels of these T cells in different stages of T1D were investigated. Th22 cells showed a positive correlation with Th17 cells in T1D patients. However, we did not find any correlation between IL-17 and IL-22 in sera. Autoantibodies were not significantly different between patients with early T1D and those who have had it for a longer duration. This study indicates that Th22 may contribute to the pathogenesis of T1D. Blockade of Th22 cells might be of clinical profit in T1D patients.






Similar content being viewed by others
References
J.A. Bluestone, K. Herold, G. Eisenbarth, Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464(7293), 1293–1300 (2010)
J.A. Emamaullee, J. Davis, S. Merani, C. Toso, J.F. Elliott, A. Thiesen, A.M. Shapiro, Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58(6), 1302–1311 (2009)
R. Jain, D.M. Tartar, R.K. Gregg, R.D. Divekar, J.J. Bell, H.H. Lee, P. Yu, J.S. Ellis, C.M. Hoeman, C.L. Franklin, H. Zaghouani, Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 205(1), 207–218 (2008)
J. Honkanen, J.K. Nieminen, R. Gao, K. Luopajarvi, H.M. Salo, J. Ilonen, M. Knip, T. Otonkoski, O. Vaarala, IL-17 immunity in human type 1 diabetes. J Immunol 185(3), 1959–1967 (2010)
P. Miossec, T. Korn, V.K. Kuchroo, Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361(9), 888–898 (2009)
S. Arif, F. Moore, K. Marks, T. Bouckenooghe, C.M. Dayan, R. Planas, M. Vives-Pi, J. Powrie, T. Tree, P. Marchetti, G.C. Huang, E.N. Gurzov, R. Pujol-Borrell, D.L. Eizirik, M. Peakman, Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 60(8), 2112–2119 (2011)
M. Ryba-Stanislawowska, M. Skrzypkowska, M. Mysliwiec, J. Mysliwska, Loss of the balance between CD4(+)Foxp3(+) regulatory T cells and CD4(+)IL17A(+) Th17 cells in patients with type 1 diabetes. Hum. Immunol. 74(6), 701–707 (2013)
S. Trifari, C.D. Kaplan, E.H. Tran, N.K. Crellin, H. Spits, Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10(8), 864–871 (2009)
M. Veldhoen, K. Hirota, A.M. Westendorf, J. Buer, L. Dumoutier, J.C. Renauld, B. Stockinger, The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191), 106–109 (2008)
L. Zhang, Y.G. Li, Y.H. Li, L. Qi, X.G. Liu, C.Z. Yuan, N.W. Hu, D.X. Ma, Z.F. Li, Q. Yang, W. Li, J.M. Li, Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One 7(4), e31000 (2012)
S. Kagami, H.L. Rizzo, J.J. Lee, Y. Koguchi, A. Blauvelt, Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest. Dermatol. 130(5), 1373–1383 (2010)
T. Liu, L. Peng, P. Yu, Y. Zhao, Y. Shi, X. Mao, W. Chen, P. Cheng, T. Wang, N. Chen, J. Zhang, X. Liu, N. Li, G. Guo, W. Tong, Y. Zhuang, Q. Zou, Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J. Clin. Immunol. 32(6), 1332–1339 (2012)
M.E. Truchetet, N.C. Brembilla, E. Montanari, Y. Allanore, C. Chizzolini, Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res. Ther. 13(5), R166 (2011)
T. Kondo, H. Takata, F. Matsuki, M. Takiguchi, Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J. Immunol. 182(4), 1794–1798 (2009)
M. Huber, S. Heink, H. Grothe, A. Guralnik, K. Reinhard, K. Elflein, T. Hunig, H.W. Mittrucker, A. Brustle, T. Kamradt, M. Lohoff, A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur. J. Immunol. 39(7), 1716–1725 (2009)
A.M. Intlekofer, A. Banerjee, N. Takemoto, S.M. Gordon, C.S. Dejong, H. Shin, C.A. Hunter, E.J. Wherry, T. Lindsten, S.L. Reiner, Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321(5887), 408–411 (2008)
D.M. Kuang, C. Peng, Q. Zhao, Y. Wu, L.Y. Zhu, J. Wang, X.Y. Yin, L. Li, L. Zheng, Tumor-activated monocytes promote expansion of IL-17-producing CD8 + T cells in hepatocellular carcinoma patients. J. Immunol. 185(3), 1544–1549 (2010)
J.S. Tzartos, M.A. Friese, M.J. Craner, J. Palace, J. Newcombe, M.M. Esiri, L. Fugger, Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172(1), 146–155 (2008)
P.C. Res, G. Piskin, O.J. de Boer, C.M. van der Loos, P. Teeling, J.D. Bos, M.B. Teunissen, Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One 5(11), e14108 (2010)
A. Saxena, S. Desbois, N. Carrie, M. Lawand, L.T. Mars, R.S. Liblau, Tc17 CD8+ T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J. Immunol. 189(6), 3140–3149 (2012)
L. Yu, K. Herold, H. Krause-Steinrauf, P.L. McGee, B. Bundy, A. Pugliese, J. Krischer, G.S. Eisenbarth, Rituximab selectively suppresses specific islet antibodies. Diabetes 60(10), 2560–2565 (2011)
T. Duhen, R. Geiger, D. Jarrossay, A. Lanzavecchia, F. Sallusto, Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10(8), 857–863 (2009)
N. Zhang, H.F. Pan, D.Q. Ye, Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol. Cell. Biochem. 353(1–2), 41–46 (2011)
H. Chen, F. Wen, X. Zhang, S.B. Su, Expression of T-helper-associated cytokines in patients with type 2 diabetes mellitus with retinopathy. Molecular vision 18, 219–226 (2012)
X.Y. Yang, H.Y. Wang, X.Y. Zhao, L.J. Wang, Q.H. Lv, Q.Q. Wang, Th22, but not Th17 Might be a Good Index to Predict the Tissue Involvement of Systemic Lupus Erythematosus. J. Clin. Immunol. 33(4), 767–774 (2013)
S. Tsai, A. Shameli, P. Santamaria, CD8 + T cells in type 1 diabetes. Adv. Immunol. 100, 79–124 (2008)
M. Huber, S. Heink, A. Pagenstecher, K. Reinhard, J. Ritter, A. Visekruna, A. Guralnik, N. Bollig, K. Jeltsch, C. Heinemann, E. Wittmann, T. Buch, O. Prazeres da Costa, A. Brustle, D. Brenner, T.W. Mak, H.W. Mittrucker, B. Tackenberg, T. Kamradt, M. Lohoff, IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Investig. 123(1), 247–260 (2013)
C. Ortega, A.S. Fernandez, J.M. Carrillo, P. Romero, I.J. Molina, J.C. Moreno, M. Santamaria, IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J. Leukoc. Biol. 86(2), 435–443 (2009)
H. Hamada, L. Garcia-Hernandez Mde, J.B. Reome, S.K. Misra, T.M. Strutt, K.K. McKinstry, A.M. Cooper, S.L. Swain, R.W. Dutton, Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182(6), 3469–3481 (2009)
C.S. Hinrichs, A. Kaiser, C.M. Paulos, L. Cassard, L. Sanchez-Perez, B. Heemskerk, C. Wrzesinski, Z.A. Borman, P. Muranski, N.P. Restifo, Type 17 CD8 + T cells display enhanced antitumor immunity. Blood 114(3), 596–599 (2009)
S. Eyerich, K. Eyerich, A. Cavani, C. Schmidt-Weber, IL-17 and IL-22: siblings, not twins. Trends Immunol. 31(9), 354–361 (2010)
E. Volpe, N. Servant, R. Zollinger, S.I. Bogiatzi, P. Hupe, E. Barillot, V. Soumelis, A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9(6), 650–657 (2008)
Y. Zheng, D.M. Danilenko, P. Valdez, I. Kasman, J. Eastham-Anderson, J. Wu, W. Ouyang, Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445(7128), 648–651 (2007)
F. Abbasi, P. Amiri, F.A. Sayahpour, S. Pirmoradi, M. Abolhalaj, B. Larijani, J.T. Bazzaz, M.M. Amoli, TGF-beta and IL-23 gene expression in unstimulated PBMCs of patients with diabetes. Endocrine 41(3), 430–434 (2012)
P.R. Mangan, L.E. Harrington, D.B. O’Quinn, W.S. Helms, D.C. Bullard, C.O. Elson, R.D. Hatton, S.M. Wahl, T.R. Schoeb, C.T. Weaver, Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441(7090), 231–234 (2006)
M. Ryba-Stanislawowska, M. Skrzypkowska, J. Mysliwska, M. Mysliwiec, The serum IL-6 profile and Treg/Th17 peripheral cell populations in patients with type 1 diabetes. Mediators Inflamm. 2013, 205284 (2013)
P.J. Bingley, Clinical applications of diabetes antibody testing. J. Clin. Endocrinol. Metab. 95(1), 25–33 (2010)
P. Achenbach, K. Koczwara, A. Knopff, H. Naserke, A.G. Ziegler, E. Bonifacio, Mature high-affinity immune responses to (pro) insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J. Clin. Invest. 114(4), 589–597 (2004)
P. Vardi, A.G. Ziegler, J.H. Mathews, S. Dib, R.J. Keller, A.T. Ricker, J.I. Wolfsdorf, R.D. Herskowitz, A. Rabizadeh, G.S. Eisenbarth et al., Concentration of insulin autoantibodies at onset of type I diabetes. Inverse log-linear correlation with age. Diabetes Care 11(9), 736–739 (1988)
S.A. Arslanian, D.J. Becker, B. Rabin, R. Atchison, M. Eberhardt, D. Cavender, J. Dorman, A.L. Drash, Correlates of insulin antibodies in newly diagnosed children with insulin-dependent diabetes before insulin therapy. Diabetes 34(9), 926–930 (1985)
J.M. Wenzlau, M. Walter, T.J. Gardner, L.M. Frisch, L. Yu, G.S. Eisenbarth, A.G. Ziegler, H.W. Davidson, J.C. Hutton, Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J. Clin. Endocrinol. Metab. 95(10), 4712–4719 (2010)
P. Achenbach, E. Bonifacio, A.J. Williams, A.G. Ziegler, E.A. Gale, P.J. Bingley, Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia 51(3), 488–492 (2008)
Acknowledgments
The study was supported by Grants from the National Natural Science Foundation of China (number 30971405, 81270897) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, X., Zheng, S., Yang, F. et al. Increased Th22 cells are independently associated with Th17 cells in type 1 diabetes. Endocrine 46, 90–98 (2014). https://doi.org/10.1007/s12020-013-0030-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0030-z