Abstract
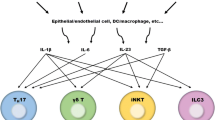
T helper cell 22 (Th22) is a new subset of T cells clearly separate from Th17 and other known T cell subsets with distinct gene expression and function. With the CCR6 + CCR4 + CCR10 + phenotype and aryl hydrocarbon receptor as the key transcription factor, Th22 subsets produce cytokines such as IL-22, whose function depends on the activation of signal transduction and activators of transcription 3. IL-22 was up-regulated in Rheumatoid arthritis, Crohn’s disease, Psoriasis, and atopic dermatitis patients whereas it was down-regulated in the serum of patients with sarcoidosis and systemic lupus erythematosus. Furthermore, it has been demonstrated that IL-22 may have promise as a potential therapeutic for chronic inflammatory diseases, and treatment with recombinant cytokine or gene therapy delivery of IL-22 may alleviate tissue destruction during inflammatory responses. In summary, Th22 cell plays an important and complicated role in inflammatory and autoimmune disease.
Similar content being viewed by others
References
Burgler S, Ouaked N, Bassin C, Basinski TM, Mantel PY, Siegmund K, Meyer N, Akdis CA, Schmidt-Weber CB (2009) Differentiation and functional analysis of human T(H)17 cells. J Allergy Clin Immunol 123(3):588–595. doi:10.1016/j.jaci.2008.12.017 595 e581–587
Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, Ring J, Traidl-Hoffmann C, Albanesi C, Cavani A (2009) IL-17 in atopic eczema: linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol 123(1):59–66. doi:10.1016/j.jaci.2008.10.031 e54
Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14(3):275–281. doi:10.1038/nm1710
Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119(12):3573–3585. doi:10.1172/JCI40202
Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F (2009) Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10(8):857–863. doi:10.1038/ni.1767
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453(7191):106–109. doi:10.1038/nature06881
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453(7191):65–71. doi:10.1038/nature06880
Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W (2007) Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445(7128):648–651. doi:10.1038/nature05505
Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, Korkalainen M, Tuomisto J, Pohjanvirta R (2005) Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: the case of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol 207(2 Suppl):43–51. doi:10.1016/j.taap.2004.12.028
Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun 1(8):488–494. doi:10.1038/sj.gene.6363716
Renauld JC (2003) Class II cytokine receptors and their ligands: key antiviral and inflammatory modulators. Nat Rev Immunol 3(8):667–676
Langer JA, Cutrone EC, Kotenko S (2004) The Class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev 15(1):33–48
Wolk K, Sabat R (2006) Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev 17(5):367–380. doi:10.1016/j.cytogfr.2006.09.001
Wolk K, Kunz S, Asadullah K, Sabat R (2002) Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol 168(11):5397–5402
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R (2004) IL-22 increases the innate immunity of tissues. Immunity 21(2):241–254. doi:10.1016/j.immuni.2004.07.007
Dumoutier L, Louahed J, Renauld JC (2000) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol 164(4):1814–1819
Dumoutier L, Van Roost E, Colau D, Renauld JC (2000) Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA 97(18):10144–10149. doi:10.1073/pnas.170291697
Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, Gurney AL (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275(40):31335–31339. doi:10.1074/jbc.M005304200
Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, Renauld JC (2002) Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277(37):33676–33682. doi:10.1074/jbc.M204204200
Witte E, Witte K, Warszawska K, Sabat R, Wolk K (2010) Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 21(5):365–379. doi:10.1016/j.cytogfr.2010.08.002
Arend WP, Dayer JM (1995) Inhibition of the production and effects of interleukin-1 and tumor necrosis factor alpha in rheumatoid arthritis. Arthritis Rheum 38(2):151–160
Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, Volk HD, Sterry W, Sabat R (2006) IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol 36(5):1309–1323. doi:10.1002/eji.200535503
Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, Nojima Y (2005) Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum 52(4):1037–1046. doi:10.1002/art.20965
Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, Matthys P (2009) Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum 60(2):390–395. doi:10.1002/art.24220
Cobrin GM, Abreu MT (2005) Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev 206:277–295. doi:10.1111/j.0105-2896.2005.00293.x
Fuss IJ, Becker C, Yang Z, Groden C, Hornung RL, Heller F, Neurath MF, Strober W, Mannon PJ (2006) Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm Bowel Dis 12(1):9–15
Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206(7):1465–1472. doi:10.1084/jem.20082683
Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y (2005) Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129(3):969–984. doi:10.1053/j.gastro.2005.06.071
Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J (2006) IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol 290(4):G827–G838. doi:10.1152/ajpgi.00513.2005
Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T (2010) Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest 90(3):496–505. doi:10.1038/labinvest.2009.147
Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, Sabat R (2007) IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 178(9):5973–5981
Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C (2010) IL-17 and IL-22: siblings, not twins. Trends Immunol 31(9):354–361. doi:10.1016/j.it.2010.06.004
Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, Sabat R (2009) IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med 87(5):523–536. doi:10.1007/s00109-009-0457-0
Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E (2009) IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123(6):1244–1252. doi:10.1016/j.jaci.2009.03.041 e1242
Schmidt-Weber CB, Akdis M, Akdis CA (2007) TH17 cells in the big picture of immunology. J Allergy Clin Immunol 120(2):247–254. doi:10.1016/j.jaci.2007.06.039
Pan HF, Ye DQ, Li XP (2008) Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol 4(7):352–353. doi:10.1038/ncprheum0815
Ciprandi G, Fenoglio D, De Amici M, Quaglini S, Negrini S, Filaci G (2008) Serum IL-17 levels in patients with allergic rhinitis. J Allergy Clin Immunol 122(3):650–651. doi:10.1016/j.jaci.2008.06.005 e652
Pan HF, Zhao XF, Yuan H, Zhang WH, Li XP, Wang GH, Wu GC, Tang XW, Li WX, Li LH, Feng JB, Hu CS, Ye DQ (2009) Decreased serum IL-22 levels in patients with systemic lupus erythematosus. Clin Chim Acta 401(1–2):179–180. doi:10.1016/j.cca.2008.11.009
Cheng F, Guo Z, Xu H, Yan D, Li Q (2009) Decreased plasma IL22 levels, but not increased IL17 and IL23 levels, correlate with disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis 68(4):604–606. doi:10.1136/ard.2008.097089
Whittington HA, Armstrong L, Uppington KM, Millar AB (2004) Interleukin-22: a potential immunomodulatory molecule in the lung. Am J Respir Cell Mol Biol 31(2):220–226. doi:10.1165/rcmb.2003-0285OC
Radaeva S, Sun R, Pan HN, Hong F, Gao B (2004) Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39(5):1332–1342. doi:10.1002/hep.20184
Kreymborg K, Etzensperger R, Dumoutier L, Haak S, Rebollo A, Buch T, Heppner FL, Renauld JC, Becher B (2007) IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J Immunol 179(12):8098–8104
Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA (2007) Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27(4):647–659. doi:10.1016/j.immuni.2007.07.023
Chang H, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R, Abe S, Toba K, Yoshida K, Elnaggar R, Minagawa S, Okura Y, Kato K, Kodama M, Maruyama H, Miyazaki J, Aizawa Y (2006) Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J Immunol 177(6):3635–3643
Pan H, Hong F, Radaeva S, Gao B (2004) Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol 1(1):43–49
Acknowledgments
The study was supported by grants from the key program of National Natural Science Foundation of China (30830089).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, N., Pan, HF. & Ye, DQ. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol Cell Biochem 353, 41–46 (2011). https://doi.org/10.1007/s11010-011-0772-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0772-y