Abstract
Aims/hypothesis
The aim of this study was to evaluate the prognostic significance of autoantibodies to IA-2β (IA2βA) in a large, well-characterised population of islet cell antibody (ICA)-positive relatives followed for 5 years in the European Nicotinamide Diabetes Intervention Trial.
Methods
Autoantibodies to insulin (IAA), glutamate decarboxylase (GADA) and IA-2 (IA2A) were measured in 549 participants at study entry, and IA2A-positive samples tested for IA2βA. First-phase insulin response (FPIR) and oral glucose tolerance were determined at baseline.
Results
Of 212 ICA/IA2A-positive participants (median age 12.1 years; 57% male), 113 developed diabetes (5 year cumulative risk 56%), and 148 were also GADA-positive and IAA-positive (4Ab-positive). IA2βA were detected in 137 (65%) ICA/IA2A-positive participants and were associated with an increased 5 year diabetes risk (IA2βA-positive 65 vs 39% in IA2βA-negative, p = 0.0002). The effect was most marked in 4Ab-positive relatives (72% vs 52%, p = 0.003). Metabolic testing further refined risk assessment. Among 101 4Ab-positive relatives with IA2βA, the 5 year risk was 94% in those with a low FPIR (vs 50% in those with a normal FPIR, p < 0.0001), and 95% in those with impaired glucose tolerance (IGT) (vs 66% in those with normal glucose tolerance, p < 0.0001). The median time to diagnosis of 4Ab/IA2βA-positive participants with a low FPIR was 1.5 years. Multivariate analysis confirmed IA2βA status, antibody number, young age, FPIR and IGT as independent determinants of risk.
Conclusions/interpretation
IA2βA are associated with a very high risk of diabetes in ICA/IA2A-positive relatives. Testing for IA2A/IA2βA compares favourably with the IVGTT in identifying a subgroup of autoantibody-positive relatives at increased risk. IA2βA determination should be added to screening protocols of future intervention trials.
Similar content being viewed by others
Introduction
Type 1 diabetes is preceded by the appearance of circulating islet autoantibodies that can be used for assessing diabetes risk in non-diabetic individuals [1]. The best established markers of progression are islet cell antibodies (ICA) and autoantibodies to insulin (IAA), the 65 kDa isoform of glutamate decarboxylase (GADA) and the protein tyrosine phosphatase-like antigen IA-2 (IA2A), and diabetes risk is related to the magnitude and maturity of these autoantibody responses [1–6]. IA2A have been associated with a high risk of progression [3], and smaller studies suggest that spreading of the IA-2 antibody response to include the homologue protein IA-2β identifies a subgroup of relatives at particularly high risk of rapid development of diabetes [1].
We therefore investigated the role of autoantibodies to IA-2β (IA2βA) in determining risk of progression to diabetes over a 5 year period in the large, well-characterised cohort of ICA-positive first-degree relatives of patients with type 1 diabetes recruited to the European Nicotinamide Diabetes Intervention Trial (ENDIT).
Methods
The protocol and outcome of ENDIT, and the role of additional immune, genetic and metabolic markers in assessing risk have previously been published [7, 8]. Inclusion criteria were ICA ≥20 Juvenile Diabetes Foundation (JDF) units in at least one sample measured in the central laboratory, with ICA ≥5 JDF units in a second sample and a non-diabetic OGTT at baseline. The protocol was approved by the research ethics committee or equivalent in each participating centre. Participants were randomised to receive either modified-release nicotinamide or placebo for 5 years, and were reviewed at baseline, 1 and 6 months after study entry and every 6 months thereafter, with an OGTT performed every 12 months. IVGTTs and OGTTs were carried out as previously described [8]. Diabetes and impaired glucose tolerance (IGT) were defined using WHO criteria.
Islet autoantibodies
GADA, IA2A and IAA were measured by radiobinding assay in the baseline samples of all 549 ENDIT participants, and considered positive if ≥97.5th percentile of 2,860 schoolchildren [2]. IA2βA were measured in IA2A-positive samples by radiobinding assay using [35S]methionine-labelled in vitro-translated recombinant human intracellular portion of IA-2β (amino acids 662–1,033 [9]).
Statistical analysis
We compared continuous variables by the Mann–Whitney U test and frequencies between groups by Fisher’s exact test, and used Spearman’s test to determine correlations. The Kaplan–Meier method was used for time-to-event survival analysis, comparing survival between groups by log-rank test. The follow-up period for each individual was calculated from the date of the baseline visit to the date of last contact or date of diagnosis of diabetes. We used Cox proportional hazards models to analyse the time-to-event outcome for individual and combined predictive markers, controlling for treatment group. The regression model included age at study entry, IA2A titre and number of islet autoantibodies as continuous variables, and presence or absence of IA2βA, IGT and first-phase insulin response (FPIR) above or below age-adjusted 10th percentile as categorical variables. Variables found to be significant at the 5% level in univariate analysis were included in a stepwise backward multivariate regression analysis. For all analyses, a two-tailed p value of 0.05 was considered significant. All statistical analyses were performed using the Statistical Package for Social Science (SPSS 14.0, Chicago, IL, USA).
Results
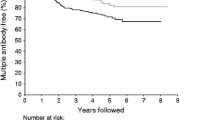
Of 549 ICA-positive family members randomised in ENDIT, 229 had IA2A ≥97.5th percentile. Baseline samples for further testing were available from 212 ICA/IA2A-positive relatives (median age 12.1 years, interquartile range [IQR] 8.4–18.1; 57% male). Of these, 113 (53%) progressed to diabetes over a median follow-up time of 2.1 years (IQR 1.0–3.3). The 5 year cumulative risk of diabetes in the IA2A-positive relatives was 56% (95% CI 49–63) (Fig. 1a).
The cumulative risk of diabetes in IA2A-positive relatives (a) in relation to presence or absence of IA2βA (p = 0.0002; b), and in IA2βA-positive relatives in relation to presence or absence of low FPIR on IVGTT (p < 0.0001; c) or IGT on OGTT (p < 0.0001; d). Thin dotted lines indicate follow-up time at 50% progression to diabetes. NGT, normal glucose tolerance
Of the 212 IA2A-positive relatives, 137 (65%) were also IA2βA-positive. IA2βA were found in 136 of 203 (67%) relatives who had IAA and/or GADA in addition to ICA and IA2A, and one of nine (11%) relatives who were positive for ICA and IA2A only (p = 0.001). The median age of IA2βA-positive relatives was 11.6 years (IQR 8.0–16.3), compared with 13.2 years (10.0–23.7) in IA2βA-negative relatives (p = 0.01). IA2βA titres were positively correlated with IA2A titre (r = 0.358, p < 0.0001), and inversely correlated with age (r = –0.223, p = 0.009). Among IA2A-positive relatives, IA2βA were strongly associated with HLA-DQ8 (p = 0.001) (data not shown).
The 5 year cumulative risk of diabetes in IA2βA-positive relatives was 65% (95% CI 57–73), compared with 39% (95% CI 28–50) in IA2βA-negative relatives (p = 0.0002; Fig. 1b). Risk in IA2βA-positive relatives could be refined by additional metabolic testing (Fig. 1c and 1d; Table 1). The impact of IA2βA on risk was most marked in relatives positive for all of the four other antibodies tested. Among 101 relatives with IA2βA in addition to ICA, GADA, IA2A and IAA, the overall risk of diabetes within 5 years was 72% (95% CI 63–81), and this increased to 94% (95% CI 86–100) and 95% (95% CI 86–100) in those with low FPIR and IGT, respectively (Table 1).
Univariate analysis showed that risk varied with IA2βA status, number of additional islet autoantibodies, age, IA2A titre, 120 min glucose in the OGTT, and FPIR. Independent determinants of risk were loss of FPIR (hazard ratio [HR] 3.1, 95% CI 2.0–4.7, p < 0.0001), presence of IGT (HR 2.8, 95% CI 1.7–4.6, p = 0.0001), IA2βA status (HR 2.1, 95% CI 1.3–3.4, p = 0.001), number of additional antibodies (HR 1.9, 95% CI 1.1–3.1, p = 0.01), and age (HR 0.965, 95% CI 0.933–0.997, p = 0.02), but not IA2A titre.
Of all ENDIT participants who developed diabetes, 79% were IA2A-positive and 59% were positive for both IA2A and IA2βA.
Discussion
Diabetes risk in islet autoantibody-positive relatives is reflected by the breadth of the autoantibody response and the target antigens involved [1, 3, 4, 8]. We have shown that autoantibodies to the IA-2β antigen detected at study entry were associated with very high diabetes risk, and that testing of IA2βA in ICA/IA2A-positive relatives could further stratify their risk. IA2βA were particularly effective in identifying relatives who progressed to disease among those with ICA plus three additional biochemical autoantibodies (IA2A, IAA and GADA). Moreover, IA2βA compared favourably with the IVGTT in identifying individuals at high risk and achieved similar sensitivity [8], while combining IA2βA determination and IVGTT with standard antibody testing allowed identification of relatives with >90% 5 year risk.
The strengths of this study are the large number of well-characterised islet autoantibody-positive relatives in our cohort, the completeness of follow-up (88% of participants), and the broad inclusion criteria used in ENDIT. A limitation is that the participants were pre-selected on the basis of confirmed high-titre ICA, which are not likely to be used to select participants for future intervention trials, and absolute risks may not reflect those in populations with lower overall risk. Participants were also selected for IA2A positivity, on the assumption that diabetes-relevant IA2βA are found only in the presence of IA2A. We are not therefore able to assess the impact of IA2βA in ICA-negative individuals or ICA-positive relatives who do not have IA2A. Further studies are required to determine whether our assumption that IA2βA testing is only necessary in IA2A-positive relatives is valid, and whether it is essential to measure the four other autoantibodies as well as IA2βA. Furthermore, the small number of relatives in this study with only two antibody markers (ICA and IA2A) means that we did not have adequate power to evaluate the additional benefit of IA2βA testing in this subgroup.
Other studies have used antibody screening and IVGTT for risk assessment in relatives [10]. We have previously suggested alternative strategies based on islet autoantibody characteristics such as titre, epitope and IgG subclass binding, and antibody affinity that could stratify diabetes risk from <10% up to >90% in smaller cohorts of relatives [1, 5, 6]. However, some of these models are labour intensive and not in general use. In contrast, IA2βA are relatively easy to measure and have recently been evaluated in an international workshop by the Diabetes Antibody Standarisation Program (DASP). When added to IA2A, IA2βA identified 53% of all ENDIT participants who progressed to diabetes, with an associated 5 year risk of 65% on the basis of testing a single sample. In comparison, a low FPIR at baseline, a more demanding and costly test, conferred a 55% 5 year risk and identified 57% of future cases [8]. Risks further increased to 72% in those IA2βA-positive relatives with four additional antibodies (65% of cases), and to 94% if FPIR was also low (at least 26% of cases).
In summary, several strategies can be used to stage pre-type 1 diabetes in antibody-positive relatives. IA2βA testing of a single sample offers a promising and flexible method of identifying those at high risk of relatively rapid progression to disease that also picks up a large proportion of future patients. Depending on the level of risk required for inclusion in a trial, a potential strategy might be to perform initial screening for IA2A (Fig. 1a), followed by measurement of IA2βA in those who are IA2A-positive (Fig. 1b), with IVGTT (Fig. 1c) or OGTT (Fig. 1d) used to identify the subgroup of IA2βA-positive relatives with the highest risk of rapid progression. Strategies based on IA2βA plus a small number of additional markers therefore provide the basis for relatively simple but highly effective identification of relatives at highest risk, and IA2βA should be included in the screening protocols for future type 1 diabetes intervention trials.
Abbreviations
- Ab:
-
antibody
- ENDIT:
-
European Nicotinamide Diabetes Intervention Trial
- FPIR:
-
first-phase insulin response
- GADA:
-
autoantibodies to glutamate decarboxylase
- HR:
-
hazard ratio
- IAA:
-
autoantibodies to insulin
- IA2A:
-
autoantibodies to IA-2
- IA2βA:
-
autoantibodies to IA-2β
- ICA:
-
islet cell antibodies
- IGT:
-
impaired glucose tolerance
- IQR:
-
interquartile range
References
Achenbach P, Warncke K, Reiter J et al (2004) Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53:384–392
Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EAM (1997) Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 46:1701–1710
Decochez K, De Leeuw IH, Keymeulen B et al (2002) IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia 45:1658–1666
Krischer JP, Cuthbertson DD, Yu L et al (2003) Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 88:103–108
Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E (2004) Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 114:589–597
Mayr A, Schlosser M, Grober N et al (2007) GAD Autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 56:1527–1533
Gale EAM, Bingley PJ, Emmett CL, Collier T (2004) European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 363:925–931
Bingley PJ, Gale EAM (2006) Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 49:881–890
Bonifacio E, Lampasona V, Bingley PJ (1998) IA-2 (islet cell antigen 512) is the primary target of humoral autoimmunity against type 1 diabetes-associated tyrosine phosphatase autoantigens. J Immunol 161:2648–2654
Diabetes Prevention Trial–Type 1 Study Group (2002) Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346:1685–1691
Acknowledgements
ENDIT received support from the European Union (grants PL920957 and PL950771), the Juvenile Diabetes Research Foundation (4-2000-943), and Novo Nordisk. Participating centres are listed in the Electronic supplementary material. P. Achenbach was supported by Juvenile Diabetes Research Foundation (11-2005-1117). The authors would like to thank N. Grober and S. Krause for technical support.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 12.9 kb)
Rights and permissions
About this article
Cite this article
Achenbach, P., Bonifacio, E., Williams, A.J.K. et al. Autoantibodies to IA-2β improve diabetes risk assessment in high-risk relatives. Diabetologia 51, 488–492 (2008). https://doi.org/10.1007/s00125-007-0912-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0912-9