Abstract
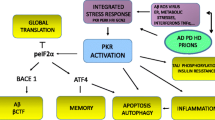
AMP-activated protein kinase (AMPK), a master regulator of cellular energy homeostasis and a central player in glucose and lipid metabolism, is potentially implicated in the pathogenesis of Alzheimer’s disease (AD). AMPK activity decreases in AD brain, indicating decreased mitochondrial biogenesis and function. Emerging evidence demonstrates that AMPK activation is a potential target for improving perturbed brain energy metabolism that is involved in the pathogenesis of AD. The roles of AMPK in the pathogenesis of AD include β-amyloid protein (Aβ) generation and tau phosphorylation. In particular, AMPK may regulate Aβ generation through modulating neuronal cholesterol and sphingomyelin levels and through regulating APP distribution in the lipid rafts. AMPK is activated by phosphorylation of Thr-172 by LKB1 complex in response to increase in the AMP/ATP ratio and by calmodulin-dependent protein kinase kinase-beta in response to elevated Ca2+ levels, which contributes to regulating Aβ generation. AMPK is a physiological tau kinase and can increase the phosphorylation of tau at Ser-262. AMPK can also directly phosphorylate tau at Thr-231 and Ser-396/404. Furthermore, AMPK activation decreases mTOR signaling activity to facilitate autophagy and promotes lysosomal degradation of Aβ. However, AMPK activation has non-neuroprotective property and may lead to detrimental outcomes, including Aβ generation and tau phosphorylation. Therefore, it is still unclear whether AMPK could serve a potential therapeutic target for AD, and hence, further studies will be needed to clarify the role of AMPK in AD.


Similar content being viewed by others
References
Ahn, J. S., Lee, J. H., Kim, J. H., & Paik, S. R. (2007). Novel method for quantitative determination of amyloid fibrils of alpha-synuclein and amyloid beta/A4 protein by using resveratrol. Analytical Biochemistry, 367, 259–265.
Alexander, A., Cai, S. L., Kim, J., Nanez, A., Sahin, M., MacLean, K. H., et al. (2010). ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proceedings of the National Academy of Sciences of the United States of America, 107, 4153–4158.
Anekonda, T. S., Wadsworth, T. L., Sabin, R., Frahler, K., Harris, C., Petriko, B., et al. (2011). Phytic acid as a potential treatment for alzheimer’s pathology: Evidence from animal and in vitro models. Journal of Alzheimer’s Disease, 23, 21–35.
Araki, A. (2010). Dementia and insulin resistance in patients with diabetes mellitus. Nihon Rinsho, 68, 569–574.
Ayasolla, K. R., Singh, A. K., & Singh, I. (2005). 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuates the expression of LPS- and Abeta peptide-induced inflammatory mediators in astroglia. Journal of Neuroinflammation, 2, 21.
Ben Sahra, I., Le Marchand-Brustel, Y., Tanti, J. F., & Bost, F. (2010). Metformin in cancer therapy: A new perspective for an old antidiabetic drug? Molecular Cancer Therapeutics, 9, 1092–1099.
Bergamini, E. (2006). Autophagy: A cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Molecular Aspects of Medicine, 27, 403–410.
Bohensky, J., Leshinsky, S., Srinivas, V., & Shapiro, I. M. (2010). Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatric Nephrology, 25, 633–642.
Boland, B., Kumar, A., Lee, S., Platt, F. M., Wegiel, J., Yu, W. H., et al. (2008). Autophagy induction and autophagosome clearance in neurons: Relationship to autophagic pathology in Alzheimer’s disease. Journal of Neuroscience, 28, 6926–6937.
Boutajangout, A., Leroy, K., Touchet, N., Authelet, M., Blanchard, V., Tremp, G., et al. (2002). Increased tau phosphorylation but absence of formation of neurofibrillary tangles in mice double transgenic for human tau and Alzheimer mutant (M146L) presenilin-1. Neuroscience Letters, 318, 29–33.
Bucht, G., Adolfsson, R., Lithner, F., & Winblad, B. (1983). Changes in blood glucose and insulin secretion in patients with senile dementia of Alzheimer type. Acta Medica Scandinavica, 213, 387–392.
Buoso, E., Lanni, C., Schettini, G., Govoni, S., & Racchi, M. (2010). Beta-Amyloid precursor protein metabolism: Focus on the functions and degradation of its intracellular domain. Pharmacological Research, 62, 308–317.
Caccamo, A., Majumder, S., Richardson, A., Strong, R., & Oddo, S. (2010). Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: Effects on cognitive impairments. Journal of Biological Chemistry, 285, 13107–13120.
Cai, Y., Wang, Q., Ling, Z., Pipeleers, D., McDermott, P., Pende, M., et al. (2008). Akt activation protects pancreatic beta cells from AMPK-mediated death through stimulation of mTOR. Biochemical Pharmacology, 75, 1981–1993.
Cammisotto, P. G., Londono, I., Gingras, D., & Bendayan, M. (2008). Control of glycogen synthase through ADIPOR1-AMPK pathway in renal distal tubules of normal and diabetic rats. American Journal of Physiology. Renal Physiology, 294, F881–F889.
Ceolotto, G., Gallo, A., Papparella, I., Franco, L., Murphy, E., Iori, E., et al. (2007). Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 2627–2633.
Chakraborty, G., Saito, M., Mao, R. F., Wang, R., & Vadasz, C. (2008). Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochemical and Biophysical Research Communications, 367, 597–602.
Chan, S. W. (2010). Family caregiving in dementia: The Asian perspective of a global problem. Dementia and Geriatric Cognitive Disorders, 30, 469–478.
Chen, M. B., McAinch, A. J., Macaulay, S. L., Castelli, L. A., O’Brien, E., Dixon, J. B., et al. (2005). Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. The Journal of Clinical Endocrinology and Metabolism, 90, 3665–3672.
Chen, L. M., Xiong, Y. S., Kong, F. L., Qu, M., Wang, Q., Chen, X. Q., et al. (2012). Neuroglobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. Journal of Neurochemistry, 120, 157–164.
Chen, Y., Zhou, K., Wang, R., Liu, Y., Kwak, Y. D., Ma, T., et al. (2009). Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America, 106, 3907–3912.
Cole, S. L., & Vassar, R. (2008). The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. Journal of Biological Chemistry, 283, 29621–29625.
Coletta, D. K., Sriwijitkamol, A., Wajcberg, E., Tantiwong, P., Li, M., Prentki, M., et al. (2009). Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: A randomised trial. Diabetologia, 52, 723–732.
Cully, M., & Downward, J. (2009). Translational responses to growth factors and stress. Biochemical Society Transactions, 37, 284–288.
Cumming, T., & Brodtmann, A. (2010). Dementia and stroke: The present and future epidemic. International Journal of Stroke, 5, 453–454.
Damjanac, M., Page, G., Ragot, S., Laborie, G., Gil, R., Hugon, J., et al. (2009). PKR, a cognitive decline biomarker, can regulate translation via two consecutive molecular targets p53 and Redd1 in lymphocytes of AD patients. Journal of Cellular and Molecular Medicine, 13, 1823–1832.
Damjanac, M., Rioux Bilan, A., Paccalin, M., Pontcharraud, R., Fauconneau, B., Hugon, J., et al. (2008). Dissociation of Akt/PKB and ribosomal S6 kinase signaling markers in a transgenic mouse model of Alzheimer’s disease. Neurobiology of Diseases, 29, 354–367.
Das, M., Das, S., Lekli, I., & Das, D. K. (2011). Caveolin induces cardioprotection through epigenetic regulation. Journal of Cellular and Molecular Medicine. doi:10.1111/j.1582-4934.2011.01372.x.
Daval, M., Ferre, P., & Foufelle, F. (2006). AMPK, an active player in the control of metabolism. Journal de la Société de Biologie, 200, 99–105.
d’Avella, D., Cicciarello, R., Gagliardi, M. E., Albiero, F., Mesiti, M., Russi, E., et al. (1994). Progressive perturbations in cerebral energy metabolism after experimental whole-brain radiation in the therapeutic range. Journal of Neurosurgery, 81, 774–779.
De Felice, F. G., Vieira, M. N., Bomfim, T. R., Decker, H., Velasco, P. T., Lambert, M. P., et al. (2009). Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Abeta oligomers. Proceedings of the National Academy of Sciences of the United States of America, 106, 1971–1976.
De Leon, M. J., George, A. E., Miller, J. D., Ferris, S. H., Klinger, A. J., Franssen, E., et al. (1988). Altered patterns of positron-emission tomography glucose metabolism in Alzheimer patients with microvascular white matter disease. American Journal of physiologic Imaging, 3, 52–53.
Dhikav, V., & Anand, K. (2011). Potential predictors of hippocampal atrophy in Alzheimer’s disease. Drugs and Aging, 28, 1–11.
Egan, D., Kim, J., Shaw, R. J., & Guan, K. L. (2011). The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy, 7, 643–644.
Enns, L. C., Pettan-Brewer, C., & Ladiges, W. (2010). Protein kinase A is a target for aging and the aging heart. Aging (Albany NY), 2, 238–243.
Erol, A. (2008). An integrated and unifying hypothesis for the metabolic basis of sporadic Alzheimer’s disease. Journal of Alzheimer’s Disease, 13, 241–253.
Fagan, A. M., Shaw, L. M., Xiong, C., Vanderstichele, H., Mintun, M. A., Trojanowski, J. Q., et al. (2011). Comparison of analytical platforms for cerebrospinal fluid measures of {beta}-amyloid 1-42, total tau, and P-tau181 for identifying Alzheimer disease amyloid plaque pathology. Archives of Neurology.
Feldman, M. E., Apsel, B., Uotila, A., Loewith, R., Knight, Z. A., Ruggero, D., et al. (2009). Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biology, 7, e38.
Finder, V. H. (2010). Alzheimer’s disease: A general introduction and pathomechanism. Journal of Alzheimer’s Disease, 22(Suppl 3), 5–19.
Flaherty, D. B., Soria, J. P., Tomasiewicz, H. G., & Wood, J. G. (2000). Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. Journal of Neuroscience Research, 62, 463–472.
Foretz, M., Hebrard, S., Leclerc, J., Zarrinpashneh, E., Soty, M., Mithieux, G., et al. (2010). Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. Journal of Clinical Investigation, 120, 2355–2369.
Foretz, M., Taleux, N., Guigas, B., Horman, S., Beauloye, C., Andreelli, F., et al. (2006). Regulation of energy metabolism by AMPK: A novel therapeutic approach for the treatment of metabolic and cardiovascular diseases. Médecine Sciences (Paris), 22, 381–388.
Gaidhu, M. P., Anthony, N. M., Patel, P., Hawke, T. J., & Ceddia, R. B. (2010). Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. American Journal of Physiology. Cell Physiology, 298, C961–C971.
Goedert, M., Hasegawa, M., Jakes, R., Lawler, S., Cuenda, A., & Cohen, P. (1997). Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Letters, 409, 57–62.
Gong, C. X., & Iqbal, K. (2008). Hyperphosphorylation of microtubule-associated protein tau: A promising therapeutic target for Alzheimer disease. Current Medicinal Chemistry, 15, 2321–2328.
Gong, C. X., Liu, F., Grundke-Iqbal, I., & Iqbal, K. (2006). Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. Journal of Alzheimers Disease, 9, 1–12.
Greco, S. J., Sarkar, S., Casadesus, G., Zhu, X., Smith, M. A., Ashford, J. W., et al. (2009a). Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neuroscience Letters, 455, 191–194.
Greco, S. J., Sarkar, S., Johnston, J. M., & Tezapsidis, N. (2009b). Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochemical and Biophysical Research Communications, 380, 98–104.
Green, A. S., Chapuis, N., Lacombe, C., Mayeux, P., Bouscary, D., & Tamburini, J. (2011). LKB1/AMPK/mTOR signaling pathway in hematological malignancies: From metabolism to cancer cell biology. Cell Cycle, 10, 2115–2120.
Grimm, M. O., Grimm, H. S., & Hartmann, T. (2007). Amyloid beta as a regulator of lipid homeostasis. Trends in Molecular Medicine, 13, 337–344.
Grosgen, S., Grimm, M. O., Friess, P., & Hartmann, T. (2010). Role of amyloid beta in lipid homeostasis. Biochimica et Biophysica Acta, 1801, 966–974.
Grotemeier, A., Alers, S., Pfisterer, S. G., Paasch, F., Daubrawa, M., Dieterle, A., et al. (2010). AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cellular Signalling, 22, 914–925.
Gupta, A., Bisht, B., & Dey, C. S. (2011). Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology, 60, 910–920.
Guze, B. H., Baxter, L. R., Jr, Schwartz, J. M., Szuba, M. P., Mazziotta, J. C., & Phelps, M. E. (1991). Changes in glucose metabolism in dementia of the Alzheimer type compared with depression: A preliminary report. Psychiatry Research, 40, 195–202.
Hahn-Windgassen, A., Nogueira, V., Chen, C. C., Skeen, J. E., Sonenberg, N., & Hay, N. (2005). Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. Journal of Biological Chemistry, 280, 32081–32089.
Han, Y. S., Zheng, W. H., Bastianetto, S., Chabot, J. G., & Quirion, R. (2004). Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase C. British Journal of Pharmacology, 141, 997–1005.
Hands, S. L., Proud, C. G., & Wyttenbach, A. (2009). mTOR’s role in ageing: Protein synthesis or autophagy? Aging (Albany NY), 1, 586–597.
Hardie, D. G. (2008). AMPK: A key regulator of energy balance in the single cell and the whole organism. International Journal of Obesity, 32(Suppl 4), S7–S12.
Hardie, D. G., & Hawley, S. A. (2001). AMP-activated protein kinase: The energy charge hypothesis revisited. Bioessays, 23, 1112–1119.
Hardie, D. G., Hawley, S. A., & Scott, J. W. (2006). AMP-activated protein kinase–development of the energy sensor concept. Journal of Physiology, 574, 7–15.
Hardie, D. G., Salt, I. P., Hawley, S. A., & Davies, S. P. (1999). AMP-activated protein kinase: An ultrasensitive system for monitoring cellular energy charge. Biochemical Journal, 338(Pt 3), 717–722.
He, H., Dong, W., & Huang, F. (2010). Anti-amyloidogenic and anti-apoptotic role of melatonin in Alzheimer disease. Current Neuropharmacology, 8, 211–217.
Hojlund, K., Mustard, K. J., Staehr, P., Hardie, D. G., Beck-Nielsen, H., Richter, E. A., et al. (2004). AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism, 286, E239–E244.
Holland, W. L., Miller, R. A., Wang, Z. V., Sun, K., Barth, B. M., Bui, H. H., et al. (2011). Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature Medicine, 17, 55–63.
Horike, N., Sakoda, H., Kushiyama, A., Ono, H., Fujishiro, M., Kamata, H., et al. (2008). AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. Journal of Biological Chemistry, 283, 33902–33910.
Hosono, K., Endo, H., Takahashi, H., Sugiyama, M., Uchiyama, T., Suzuki, K., et al. (2010). Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Molecular Carcinogenesis, 49, 662–671.
Hsueh, W. (2006). Genetic discoveries as the basis of personalized therapy: Rosiglitazone treatment of Alzheimer’s disease. Pharmacogenomics Journal, 6, 222–224.
Hu, Y., He, S., & Wang, J. (2001). Diagnostic value of tau in cerebrospinal fluid in Alzheimer disease. Zhonghua Yi Xue Za Zhi, 81, 1377–1379.
Hu, W. T., Parisi, J. E., Knopman, D. S., Boeve, B. F., Dickson, D. W., Ahlskog, J. E., et al. (2007). Clinical features and survival of 3R and 4R tauopathies presenting as behavioral variant frontotemporal dementia. Alzheimer Disease and Associated Disorders, 21, S39–S43.
Imai, K., Inukai, K., Ikegami, Y., Awata, T., & Katayama, S. (2006). LKB1, an upstream AMPK kinase, regulates glucose and lipid metabolism in cultured liver and muscle cells. Biochemical and Biophysical Research Communications, 351, 595–601.
Inoki, K., Ouyang, H., Zhu, T., Lindvall, C., Wang, Y., Zhang, X., et al. (2006). TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell, 126, 955–968.
Jaboin, J. J., Shinohara, E. T., Moretti, L., Yang, E. S., Kaminski, J. M., & Lu, B. (2007). The role of mTOR inhibition in augmenting radiation induced autophagy. Technology in Cancer Research & Treatment, 6, 443–447.
Janson, J., Laedtke, T., Parisi, J. E., O’Brien, P., Petersen, R. C., & Butler, P. C. (2004). Increased risk of type 2 diabetes in Alzheimer disease. Diabetes, 53, 474–481.
Jegga, A. G., Schneider, L., Ouyang, X., & Zhang, J. (2011). Systems biology of the autophagy-lysosomal pathway. Autophagy, 7, 477–489.
Jornayvaz, F. R., & Shulman, G. I. (2010). Regulation of mitochondrial biogenesis. Essays Biochemistry, 47, 69–84.
Ju, T. C., Chen, H. M., Lin, J. T., Chang, C. P., Chang, W. C., Kang, J. J., et al. (2011). Nuclear translocation of AMPK-{alpha}1 potentiates striatal neurodegeneration in Huntington’s disease. Journal of Cell Biology, 194, 209–227.
Kalaria, R. N., & Harik, S. I. (1989). Reduced glucose transporter at the blood–brain barrier and in cerebral cortex in Alzheimer disease. Journal of Neurochemistry, 53, 1083–1088.
Kapogiannis, D., & Mattson, M. P. (2011). Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurology, 10, 187–198.
Kemp, B. E., Stapleton, D., Campbell, D. J., Chen, Z. P., Murthy, S., Walter, M., et al. (2003). AMP-activated protein kinase, super metabolic regulator. Biochemical Society Transactions, 31, 162–168.
Kickstein, E., Krauss, S., Thornhill, P., Rutschow, D., Zeller, R., Sharkey, J., et al. (2010). Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the United States of America, 107, 21830–21835.
Kim, J., Kundu, M., Viollet, B., & Guan, K. L. (2011). AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology, 13, 132–141.
Kim, A. S., Miller, E. J., & Young, L. H. (2009). AMP-activated protein kinase: A core signalling pathway in the heart. Acta Physiology (Oxf), 196, 37–53.
King, T. D., Song, L., & Jope, R. S. (2006). AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochemical Pharmacology, 71, 1637–1647.
Knopman, D. (2001). Cerebrospinal fluid beta-amyloid and tau proteins for the diagnosis of Alzheimer disease. Archives of Neurology, 58, 349–350.
Kohjima, M., Higuchi, N., Kato, M., Kotoh, K., Yoshimoto, T., Fujino, T., et al. (2008). SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. International Journal of Molecular Medicine, 21, 507–511.
Kola, B., Grossman, A. B., & Korbonits, M. (2008). The role of AMP-activated protein kinase in obesity. Frontiers of Hormone Research, 36, 198–211.
Koren, I., Reem, E., & Kimchi, A. (2010). DAP1, a novel substrate of mTOR, negatively regulates autophagy. Current Biology, 20, 1093–1098.
Koss, E., Friedland, R. P., Ober, B. A., & Jagust, W. J. (1985). Differences in lateral hemispheric asymmetries of glucose utilization between early- and late-onset Alzheimer-type dementia. American Journal of Psychiatry, 142, 638–640.
Kudchodkar, S. B., Del Prete, G. Q., Maguire, T. G., & Alwine, J. C. (2007). AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. Journal of Virology, 81, 3649–3651.
Kumar, S. H., & Rangarajan, A. (2009). Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation. Journal of Virology, 83, 8565–8574.
Kwon, K. J., Kim, H. J., Shin, C. Y., & Han, S. H. (2010). Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating AMP-activated protein kinase pathways. Journal of Clinical Neurology, 6, 127–137.
Labuzek, K., Liber, S., Gabryel, B., & Okopien, B. (2010). AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside) increases the production of toxic molecules and affects the profile of cytokines release in LPS-stimulated rat primary microglial cultures. Neurotoxicology, 31, 134–146.
Landreth, G. (2006). PPARgamma agonists as new therapeutic agents for the treatment of Alzheimer’s disease. Experimental Neurology, 199, 245–248.
Leclerc, S., Garnier, M., Hoessel, R., Marko, D., Bibb, J. A., Snyder, G. L., et al. (2001). Indirubins inhibit glycogen synthase kinase-3 beta and CDK5/p25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer’s disease. A property common to most cyclin-dependent kinase inhibitors? Journal of Biological Chemistry, 276, 251–260.
Lee, J. W., Park, S., Takahashi, Y., & Wang, H. G. (2010). The association of AMPK with ULK1 regulates autophagy. PLoS One, 5, e15394.
Li, J., Benashski, S., & McCullough, L. D. (2011). Post-stroke hypothermia provides neuroprotection through inhibition of AMP-activated protein kinase. Journal of Neurotrauma, 28, 1281–1288.
Li, J., Benashski, S. E., Siegel, C., Liu, F., & McCullough, L. D. (2010). Adenosine monophosphate activated protein kinase inhibition is protective in both sexes after experimental stroke. Neuroscience Letters, 482, 62–65.
Li, J., & McCullough, L. D. (2010). Effects of AMP-activated protein kinase in cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism, 30, 480–492.
Li, J., Zeng, Z., Viollet, B., Ronnett, G. V., & McCullough, L. D. (2007). Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke, 38, 2992–2999.
Lin, J. N., Lin, V. C., Rau, K. M., Shieh, P. C., Kuo, D. H., Shieh, J. C., et al. (2010). Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. Journal of Agricultural and Food Chemistry, 58, 1584–1592.
Ling, D., & Salvaterra, P. M. (2011). Brain aging and Abeta neurotoxicity converge via deterioration in autophagy-lysosomal system: A conditional drosophila model linking Alzheimer’s neurodegeneration with aging. Acta Neuropathologica, 121, 183–191.
Littler, D. R., Walker, J. R., Davis, T., Wybenga-Groot, L. E., Finerty, P. J., Jr, Newman, E., et al. (2010). A conserved mechanism of autoinhibition for the AMPK kinase domain: ATP-binding site and catalytic loop refolding as a means of regulation. Acta Crystallographica. Section F, Structural Biology and Crystallization Communications, 66, 143–151.
Liu, F., Liang, Z., & Gong, C. X. (2006). Hyperphosphorylation of tau and protein phosphatases in Alzheimer disease. Panminerva Medica, 48, 97–108.
Lu, J., Wu, D. M., Zheng, Y. L., Hu, B., Zhang, Z. F., Shan, Q., et al. (2010). Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. Journal of Pathology, 222, 199–212.
Mair, W., Morantte, I., Rodrigues, A. P., Manning, G., Montminy, M., Shaw, R. J., et al. (2011). Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature, 470, 404–408.
Mancuso, M., Orsucci, D., LoGerfo, A., Calsolaro, V., & Siciliano, G. (2010). Clinical features and pathogenesis of Alzheimer’s disease: Involvement of mitochondria and mitochondrial DNA. Advances in Experimental Medicine and Biology, 685, 34–44.
Mao, K., & Klionsky, D. J. (2011). AMPK activates autophagy by phosphorylating ULK1. Circulation Research, 108, 787–788.
Marambaud, P., Zhao, H., & Davies, P. (2005). Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. Journal of Biological Chemistry, 280, 37377–37382.
Marshall, S. (2006). Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Science’s STKE, 2006, re7.
Martin, D. E., & Hall, M. N. (2005). The expanding TOR signaling network. Current Opinion in Cell Biology, 17, 158–166.
Martinez de Morentin, P. B., Gonzalez, C. R., & Lopez, M. (2010). AMP-activated protein kinase: ‘A cup of tea’ against cholesterol-induced neurotoxicity. Journal of Pathology, 222, 329–334.
Mattson, M. P., Pedersen, W. A., Duan, W., Culmsee, C., & Camandola, S. (1999). Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. Annals of the New York Academy of Sciences, 893, 154–175.
McCullough, L. D., Zeng, Z., Li, H., Landree, L. E., McFadden, J., & Ronnett, G. V. (2005). Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. Journal of Biological Chemistry, 280, 20493–20502.
McNamara, D., Horwitz, B., Grady, C. L., & Rapoport, S. I. (1987). Topographical analysis of glucose metabolism, as measured with positron emission tomography, in dementia of the Alzheimer type: Use of linear histograms. International Journal of Neuroscience, 36, 89–97.
Miranda, N., Tovar, A. R., Palacios, B., & Torres, N. (2007). AMPK as a cellular energy sensor and its function in the organism. Revista de Investigacion Clinica, 59, 458–469.
Misra, P. (2008). AMP activated protein kinase: A next generation target for total metabolic control. Expert Opinion on Therapeutic Targets, 12, 91–100.
Morrison, A., Yan, X., Tong, C., & Li, J. (2011). Acute rosiglitazone treatment is cardioprotective against ischemia/reperfusion injury by modulating AMPK, Akt, and JNK signaling in non-diabetic mice. American Journal of Physiology. Heart and Circulatory Physiology, 301, H895–H902.
Mulder, C., Verwey, N. A., van der Flier, W. M., Bouwman, F. H., Kok, A., van Elk, E. J., et al. (2010). Amyloid-beta(1–42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clinical Chemistry, 56, 248–253.
Nemoto, T., Satoh, S., Maruta, T., Kanai, T., Yoshikawa, N., Miyazaki, S., et al. (2010). Homologous posttranscriptional regulation of insulin-like growth factor-I receptor level via glycogen synthase kinase-3beta and mammalian target of rapamycin in adrenal chromaffin cells: Effect on tau phosphorylation. Neuropharmacology, 58, 1097–1108.
Newman, M., Musgrave, I. F., & Lardelli, M. (2007). Alzheimer disease: Amyloidogenesis, the presenilins and animal models. Biochimica et Biophysica Acta, 1772, 285–297.
Oh, K. J., Park, J., Lee, S. Y., Hwang, I., Kim, J. B., Park, T. S., et al. (2011). Atypical antipsychotic drugs perturb AMPK-dependent regulation of hepatic lipid metabolism. American Journal of Physiology. Endocrinology and Metabolism, 300, E624–E632.
Olcese, J. M., Cao, C., Mori, T., Mamcarz, M. B., Maxwell, A., Runfeldt, M. J., et al. (2009). Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. Journal of Pineal Research, 47, 82–96.
Pappolla, M., Bozner, P., Soto, C., Shao, H., Robakis, N. K., Zagorski, M., et al. (1998). Inhibition of Alzheimer beta-fibrillogenesis by melatonin. Journal of Biological Chemistry, 273, 7185–7188.
Pei, J. J., & Hugon, J. (2008). mTOR-dependent signalling in Alzheimer’s disease. Journal of Cellular and Molecular Medicine, 12, 2525–2532.
Pelech, S.L. (1995). Networking with proline-directed protein kinases implicated in tau phosphorylation. Neurobiology of Aging, 16, 247–256; discussion 257–261.
Poels, J., Spasic, M. R., Callaerts, P., & Norga, K. K. (2009). Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays, 31, 944–952.
Pous, C., & Codogno, P. (2011). Lysosome positioning coordinates mTORC1 activity and autophagy. Nature Cell Biology, 13, 342–344.
Qin, L., Wang, Z., Tao, L., & Wang, Y. (2010). ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy, 6, 239–247.
Ravikumar, B., & Rubinsztein, D. C. (2006). Role of autophagy in the clearance of mutant huntingtin: A step towards therapy? Molecular Aspects of Medicine, 27, 520–527.
Reynolds, C. H., Garwood, C. J., Wray, S., Price, C., Kellie, S., Perera, T., et al. (2008). Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cgamma1, Grb2, and Src family kinases. Journal of Biological Chemistry, 283, 18177–18186.
Roggo, S. (2002). Inhibition of BACE, a promising approach to Alzheimer’s disease therapy. Current Topics in Medicinal Chemistry, 2, 359–370.
Ronnett, G. V., Ramamurthy, S., Kleman, A. M., Landree, L. E., & Aja, S. (2009). AMPK in the brain: Its roles in energy balance and neuroprotection. Journal of Neurochemistry, 109(Suppl 1), 17–23.
Rosenbluth, J. M., & Pietenpol, J. A. (2009). mTOR regulates autophagy-associated genes downstream of p73. Autophagy, 5, 114–116.
Rosner, M., Hanneder, M., Siegel, N., Valli, A., Fuchs, C., & Hengstschlager, M. (2008). The mTOR pathway and its role in human genetic diseases. Mutation Research, 659, 284–292.
Rossner, S., Lange-Dohna, C., Zeitschel, U., & Perez-Polo, J. R. (2005). Alzheimer’s disease beta-secretase BACE1 is not a neuron-specific enzyme. Journal of Neurochemistry, 92, 226–234.
Saito, M., Chakraborty, G., Mao, R. F., Wang, R., Cooper, T. B., & Vadasz, C. (2007). Ethanol alters lipid profiles and phosphorylation status of AMP-activated protein kinase in the neonatal mouse brain. Journal of Neurochemistry, 103, 1208–1218.
Salminen, A., Kaarniranta, K., Haapasalo, A., Soininen, H., & Hiltunen, M. (2011). AMP-activated protein kinase: A potential player in Alzheimer’s disease. Journal of Neurochemistry, 118, 460–474.
Santomauro Junior, A. C., Ugolini, M. R., Santomauro, A. T., & Souto, R. P. (2008). Metformin and AMPK: An old drug and a new enzyme in the context of metabolic syndrome. Arquivos Brasileiros de Endocrinologia e Metabologia, 52, 120–125.
Sato, T., Hanyu, H., Akai, T., Takasaki, A., Sakurai, H., & Iwamoto, T. (2008). A patient with early Alzheimer’s disease who showed improvement of cognitive function and cerebral perfusion by combined therapy of nilvadipine and PPAR gamma agonists. Nihon Ronen Igakkai Zasshi, 45, 428–433.
Sato, N., Takeda, S., Uchio-Yamada, K., Ueda, H., Fujisawa, T., Rakugi, H., et al. (2011). Role of insulin signaling in the interaction between Alzheimer disease and diabetes mellitus: A missing link to therapeutic potential. Current Aging Science, 4, 118–127.
Savaskan, E., Olivieri, G., Meier, F., Seifritz, E., Wirz-Justice, A., & Muller-Spahn, F. (2003). Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology, 49, 380–383.
Sawamura, N., Ko, M., Yu, W., Zou, K., Hanada, K., Suzuki, T., et al. (2004). Modulation of amyloid precursor protein cleavage by cellular sphingolipids. Journal of Biological Chemistry, 279, 11984–11991.
Scales, T. M., Derkinderen, P., Leung, K. Y., Byers, H. L., Ward, M. A., Price, C., et al. (2011). Tyrosine phosphorylation of tau by the SRC family kinases lck and fyn. Molecular Neurodegeneration, 6, 12.
Schubert, D. (2005). Glucose metabolism and Alzheimer’s disease. Ageing Research Reviews, 4, 240–257.
Sengupta, A., Grundke-Iqbal, I., & Iqbal, K. (2006). Regulation of phosphorylation of tau by protein kinases in rat brain. Neurochemical Research, 31, 1473–1480.
Shacka, J. J., Roth, K. A., & Zhang, J. (2008). The autophagy-lysosomal degradation pathway: Role in neurodegenerative disease and therapy. Frontiers in Bioscience, 13, 718–736.
Singh, T. J., Grundke-Iqbal, I., McDonald, B., & Iqbal, K. (1994). Comparison of the phosphorylation of microtubule-associated protein tau by non-proline dependent protein kinases. Molecular and Cellular Biochemistry, 131, 181–189.
Singh, T. J., Haque, N., Grundke-Iqbal, I., & Iqbal, K. (1995). Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline-dependent protein kinases and GSK-3. FEBS Letters, 358, 267–272.
Sisodia, S. S., & Price, D. L. (1995). Role of the beta-amyloid protein in Alzheimer’s disease. FASEB Journal, 9, 366–370.
Song, G. Y., Gao, Y., Wang, C., Hu, S. G., Wang, J., Qu, D. M., et al. (2010). Rosiglitazone reduces fatty acid translocase and increases AMPK in skeletal muscle in aged rats: A possible mechanism to prevent high-fat-induced insulin resistance. Chinese Medical Journal, 123, 2384–2391.
Spilman, P., Podlutskaya, N., Hart, M. J., Debnath, J., Gorostiza, O., Bredesen, D., et al. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One, 5, e9979.
Steinberg, G. R., & Kemp, B. E. (2009). AMPK in health and disease. Physiological Reviews, 89, 1025–1078.
Szelies, B., Herholz, K., Pawlik, G., Beil, C., Wienhard, K., & Heiss, W. D. (1986). Cerebral glucose metabolism in presenile dementia of the Alzheimer type–follow-up of therapy with muscarinergic choline agonists. Fortschritte der Neurologie-Psychiatrie, 54, 364–373.
Takeda, S., Sato, N., Rakugi, H., & Morishita, R. (2011). Molecular mechanisms linking diabetes mellitus and Alzheimer disease: Beta-amyloid peptide, insulin signaling, and neuronal function. Molecular Biosystems, 7, 1822–1827.
Tapiola, T., Alafuzoff, I., Herukka, S. K., Parkkinen, L., Hartikainen, P., Soininen, H., et al. (2009). Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of Neurology, 66, 382–389.
Thornton, C., Bright, N. J., Sastre, M., Muckett, P. J., & Carling, D. (2011). AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochemical Journal, 434, 503–512.
Tong, X., Smith, K. A., & Pelling, J. C. (2012). Apigenin, a chemopreventive bioflavonoid, induces AMP-activated protein kinase activation in human keratinocytes. Molecular Carcinogenesis, 51, 268–279.
Towler, M. C., & Hardie, D. G. (2007). AMP-activated protein kinase in metabolic control and insulin signaling. Circulation Research, 100, 328–341.
Tschape, J. A., Hammerschmied, C., Muhlig-Versen, M., Athenstaedt, K., Daum, G., & Kretzschmar, D. (2002). The neurodegeneration mutant lochrig interferes with cholesterol homeostasis and Appl processing. EMBO Journal, 21, 6367–6376.
Vassar, R. (2002). Beta-secretase (BACE) as a drug target for Alzheimer’s disease. Advanced Drug Delivery Reviews, 54, 1589–1602.
Vassar, R. (2004). BACE1: The beta-secretase enzyme in Alzheimer’s disease. Journal of Molecular Neuroscience, 23, 105–114.
Vassar, R., Bennett, B. D., Babu-Khan, S., Kahn, S., Mendiaz, E. A., Denis, P., et al. (1999). Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science, 286, 735–741.
Vila, L., Roglans, N., Perna, V., Sanchez, R. M., Vazquez-Carrera, M., Alegret, M., et al. (2011). Liver AMP/ATP ratio and fructokinase expression are related to gender differences in AMPK activity and glucose intolerance in rats ingesting liquid fructose. The Journal of Nutritional Biochemistry, 22, 741–751.
Vingtdeux, V., Chandakkar, P., Zhao, H., d’Abramo, C., Davies, P., & Marambaud, P. (2011a). Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB Journal, 25, 219–231.
Vingtdeux, V., Chandakkar, P., Zhao, H., Davies, P., & Marambaud, P. (2011b). Small-molecule activators of AMPK, RSVA314 and RSVA405, inhibit adipogenesis. Molecular Medicine, 17, 1022–1030.
Vingtdeux, V., Davies, P., Dickson, D. W., & Marambaud, P. (2011b). AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathologica, 121, 337–349.
Vingtdeux, V., Dreses-Werringloer, U., Zhao, H., Davies, P., & Marambaud, P. (2008). Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neuroscience, 9(Suppl 2), S6.
Vingtdeux, V., Giliberto, L., Zhao, H., Chandakkar, P., Wu, Q., Simon, J. E., et al. (2010). AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. Journal of Biological Chemistry, 285, 9100–9113.
Viollet, B., & Andreelli, F. (2011). AMP-activated protein kinase and metabolic control. Handbook of Experimental Pharmacology, 203, 303–330.
Viollet, B., Lantier, L., Devin-Leclerc, J., Hebrard, S., Amouyal, C., Mounier, R., et al. (2009). Targeting the AMPK pathway for the treatment of type 2 diabetes. Frontiers in Bioscience, 14, 3380–3400.
Vogel, J., Anand, V. S., Ludwig, B., Nawoschik, S., Dunlop, J., & Braithwaite, S. P. (2009). The JNK pathway amplifies and drives subcellular changes in tau phosphorylation. Neuropharmacology, 57, 539–550.
Wang, Y., Martinez-Vicente, M., Kruger, U., Kaushik, S., Wong, E., Mandelkow, E. M., et al. (2009). Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Human Molecular Genetics, 18, 4153–4170.
Wang, J. Z., & Wang, Z. F. (2006). Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacologica Sinica, 27, 41–49.
Watanabe, R., Wei, L., & Huang, J. (2011). mTOR signaling, function, novel inhibitors, and therapeutic targets. Journal of Nuclear Medicine, 52, 497–500.
Watson, G. S., & Craft, S. (2003). The role of insulin resistance in the pathogenesis of Alzheimer’s disease: Implications for treatment. CNS Drugs, 17, 27–45.
Williamson, R., Scales, T., Clark, B. R., Gibb, G., Reynolds, C. H., Kellie, S., et al. (2002). Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: Involvement of Src family protein kinases. Journal of Neuroscience, 22, 10–20.
Won, J. S., Im, Y. B., Kim, J., Singh, A. K., & Singh, I. (2010). Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochemical and Biophysical Research Communications, 399, 487–491.
Wood, J. G., Mirra, S. S., Pollock, N. J., & Binder, L. I. (1986). Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proceedings of the National Academy of Sciences of the United States of America, 83, 4040–4043.
Xu, M., Lai, M. T., Huang, Q., DiMuzio-Mower, J., Castro, J. L., Harrison, T., et al. (2002). Gamma-Secretase: Characterization and implication for Alzheimer disease therapy. Neurobiology of Aging, 23, 1023–1030.
Yang, H., Yang, J. C., & Guan, Y. F. (2009). Role of AMPK in glucose and lipid metabolisms. Sheng Li Ke Xue Jin Zhan, 40, 249–252.
Zhang, L., Jouret, F., Rinehart, J., Sfakianos, J., Mellman, I., Lifton, R. P., et al. (2011). AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3beta (GSK-3beta) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. Journal of Biological Chemistry, 286, 16879–16890.
Zhou, G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., et al. (2001). Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation, 108, 1167–1174.
Zhuang, Y., & Miskimins, W. K. (2008). Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. Journal of molecular signaling, 3, 18.
Ziani-Cherif, C., Mostefa-Kara, B., & Brixi-Gormat, F. Z. (2006). Gamma-secretase as a pharmacological target in Alzheimer disease research: When, why and how? Current Pharmaceutical Design, 12, 4313–4335.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (81070878/H0902) to Dr. Bin Zhao.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, Z., Yan, LJ., Li, K. et al. Roles of AMP-activated Protein Kinase in Alzheimer’s Disease. Neuromol Med 14, 1–14 (2012). https://doi.org/10.1007/s12017-012-8173-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-012-8173-2