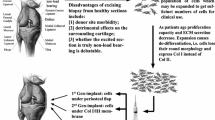
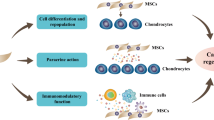
Abstract
Cartilage injuries following trauma create a puzzling clinical scenario. The finite reparative potential of articular cartilage is well known, and injuries are associated with an increased risk of osteoarthritis. Cell-based therapies have spotlighted chondrocytes and mesenchymal stromal cells (MSCs) as the functional unit of articular cartilage and the progenitor cells, respectively. The available clinical treatments cannot reproduce the biomechanical properties of articular cartilage and call for continuous investigations into alternative approaches. Co-cultures of chondrocytes and MSCs are an attractive in vitro system to step closer to the in vivo multicellular environment’s complexity. Research on the mechanisms of interaction between both cell types will reveal essential cues to understand cartilage regeneration. This review describes the latest discoveries on these interactions, along with advantages and main challenges in vitro and in vivo. The successful clinical translation of in vitro studies requires establishing rigorous standards and clinically relevant research models and an organ-targeting therapeutic strategy.
Graphical abstract





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Bhosale, A. M., & Richardson, J. B. (2008). Articular cartilage: structure, injuries and review of management. British Medical Bulletin, 87(1), 77–95. https://doi.org/10.1093/bmb/ldn025.
Zhang, Y., & Jordan, J. M. (2010). Epidemiology of osteoarthritis. Clinics in Geriatric Medicine, 26(3), 355–369. https://doi.org/10.1016/j.cger.2010.03.001.
Parkinson, L., Waters, D. L., & Franck, L. (2017). Systematic review of the impact of osteoarthritis on health outcomes for comorbid disease in older people. Osteoarthritis and Cartilage, 25(11), 1751–1770. https://doi.org/10.1016/j.joca.2017.07.008.
Hiligsmann, M., et al. (2013). Health economics in the field of osteoarthritis: An Expert’s consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Seminars in Arthritis and Rheumatism, 43(3), 303–313. https://doi.org/10.1016/j.semarthrit.2013.07.003.
Armiento, A. R., Alini, M., & Stoddart, M. J. (2019). Articular fibrocartilage - Why does hyaline cartilage fail to repair? Advanced Drug Delivery Reviews, 146, 289–305. https://doi.org/10.1016/j.addr.2018.12.015.
Steadman, J. R., Rodkey, W. G., & Briggs, K. K. (2010). Microfracture: Its history and experience of the developing surgeon. Cartilage, 1(2), 78–86. https://doi.org/10.1177/1947603510365533.
Bekkers, J. E. J., Inklaar, M., & Saris, D. B. F. (2009). Treatment selection in articular cartilage lesions of the knee: A systematic review. The American Journal of Sports Medicine, 37(1_suppl), 148–155. https://doi.org/10.1177/0363546509351143.
Brittberg, M., et al. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine, 331(14), 889–895. https://doi.org/10.1056/NEJM199410063311401.
Cherubino, P., et al. (2003). Autologous chondrocyte implantation using a bilayer collagen membrane: A preliminary report. Journal of Orthopaedic Surgery, 11(1), 10–15. https://doi.org/10.1177/230949900301100104.
Gursoy, S., et al. (2019). Factors influencing the results in matrix-associated autologous chondrocyte implantation: A 2–5 year follow-up study. Journal of Clinical Medicine Research, 11(2), 137–144. https://doi.org/10.14740/jocmr3711.
Niemeyer, P., et al. (2016). The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the Knee: A randomized clinical trial. The American Journal of Sports Medicine, 44(8), 2005–2014. https://doi.org/10.1177/0363546516646092.
Grevenstein, D., et al. (2020). Excellent histological results in terms of articular cartilage regeneration after spheroid-based autologous chondrocyte implantation (ACI). Knee Surgery, Sports Traumatology, Arthroscopy. https://doi.org/10.1007/s00167-020-05976-9.
Eschen, C., et al. (2020). Clinical outcome is significantly better with spheroid-based autologous chondrocyte implantation manufactured with more stringent cell culture criteria. Osteoarthritis and Cartilage Open, 2(1), 100033. https://doi.org/10.1016/j.ocarto.2020.100033.
Mistry, H., et al. (2017). Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technology Assessment, 21(6), 1–294. https://doi.org/10.3310/hta21060.
Hunziker, E. B., Quinn, T. M., & Häuselmann, H. J. (2002). Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis and Cartilage, 10(7), 564–572. https://doi.org/10.1053/joca.2002.0814.
Armiento, A. R., et al. (2018). Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomaterialia, 65, 1–20. https://doi.org/10.1016/j.actbio.2017.11.021.
Benya, P. (1982). Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell, 30(1), 215–224. https://doi.org/10.1016/0092-8674(82)90027-7.
Darling, E. M., et al. (2009). Mechanical properties and gene expression of chondrocytes on micropatterned substrates following dedifferentiation in monolayer. Cellular and Molecular Bioengineering, 2(3), 395–404. https://doi.org/10.1007/s12195-009-0077-3.
Barbero, A., et al. (2003). Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis and Rheumatism, 48(5), 1315–1325. https://doi.org/10.1002/art.10950.
Sanchez, C., et al. (2017). Chondrocyte secretome: a source of novel insights and exploratory biomarkers of osteoarthritis. Osteoarthritis and Cartilage, 25(8), 1199–1209. https://doi.org/10.1016/j.joca.2017.02.797.
van der Kraan, P. M. (2017). The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nature Reviews Rheumatology, 13(3), 155–163. https://doi.org/10.1038/nrrheum.2016.219.
Zhong, L., et al. (2015). The regulatory role of signaling crosstalk in hypertrophy of MSCs and human articular chondrocytes. International Journal of Molecular Sciences, 16(8), 19225–19247. https://doi.org/10.3390/ijms160819225.
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650. https://doi.org/10.1002/jor.1100090504.
Dominici, M., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. https://doi.org/10.1080/14653240600855905.
Pittenger, M. F. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147. https://doi.org/10.1126/science.284.5411.143.
Mushahary, D., et al. (2018). Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry Part A, 93(1), 19–31. https://doi.org/10.1002/cyto.a.23242.
Johnstone, B., et al. (1998). In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research, 238(1), 265–272. https://doi.org/10.1006/excr.1997.3858.
Ripmeester, E. G. J., et al. (2018). Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Frontiers in Bioengineering and Biotechnology, 6, 18–18. https://doi.org/10.3389/fbioe.2018.00018.
Mets, T., & Verdonk, G. (1981). In vitro aging of human bone marrow derived stromal cells. Mechanisms of Ageing and Development, 16(1), 81–89. https://doi.org/10.1016/0047-6374(81)90035-X.
Colter, D. C., Sekiya, I., & Prockop, D. J. (2001). Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 7841-5. https://doi.org/10.1073/pnas.141221698.
Andrzejewska, A., Lukomska, B., & Janowski, M. (2019). Concise review: Mesenchymal stem cells: from roots to boost. Stem Cells, 37(7), 855–864. https://doi.org/10.1002/stem.3016.
Park, J. S., et al. (2011). Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-beta3. Biomaterials, 32(32), 8139–8149. https://doi.org/10.1016/j.biomaterials.2011.07.043.
Han, Y., et al. (2019). Mesenchymal stem cells for regenerative medicine. Cells, 8(8), 886. https://doi.org/10.3390/cells8080886.
Horwitz, E. M., et al. (2005). Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy, 7(5), 393–395. https://doi.org/10.1080/14653240500319234.
Kook, Y.-M., et al. (2017). Design of biomimetic cellular scaffolds for co-culture system and their application. Journal of Tissue Engineering, 8, 204173141772464. https://doi.org/10.1177/2041731417724640.
Goers, L., Freemont, P., & Polizzi, K. M. (2014). Co-culture systems and technologies: taking synthetic biology to the next level. Journal of The Royal Society Interface, 11(96), 20140065. https://doi.org/10.1098/rsif.2014.0065.
Zhang, Y., et al. (2018). Co-culture systems-based strategies for articular cartilage tissue engineering. Journal of Cellular Physiology, 233(3), 1940–1951. https://doi.org/10.1002/jcp.26020.
Levorson, E. J., et al. (2014). Cell-derived polymer/extracellular matrix composite scaffolds for cartilage regeneration, part 1: investigation of cocultures and seeding densities for improved extracellular matrix deposition. Tissue Engineering Part C: Methods, 20(4), 340–357. https://doi.org/10.1089/ten.tec.2013.0286.
Meretoja, V. V., et al. (2012). Enhanced chondrogenesis in co-cultures with articular chondrocytes and mesenchymal stem cells. Biomaterials, 33(27), 6362–6369. https://doi.org/10.1016/j.biomaterials.2012.05.042.
Aung, A., et al. (2011). Osteoarthritic chondrocyte-secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis & Rheumatism, 63(1), 148–158. https://doi.org/10.1002/art.30086.
Prasadam, I., et al. (2018). Mixed cell therapy of bone marrow-derived mesenchymal stem cells and articular cartilage chondrocytes ameliorates osteoarthritis development. Laboratory Investigation, 98(1), 106–116. https://doi.org/10.1038/labinvest.2017.117.
Fischer, J., et al. (2010). Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis & Rheumatism, 62(9), 2696–2706. https://doi.org/10.1002/art.27565.
Zhao, Z., et al. (2018). Co-implantation of bone marrow mesenchymal stem cells and chondrocytes increase the viability of chondrocytes in rat osteo-chondral defects. Oncology Letters. https://doi.org/10.3892/ol.2018.8195.
Wang, Y., et al. (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature Immunology, 15(11), 1009–1016. https://doi.org/10.1038/ni.3002.
Ichiseki, T., et al. (2018). Intraarticularly-injected mesenchymal stem cells stimulate anti-inflammatory molecules and inhibit pain related protein and chondrolytic enzymes in a monoiodoacetate-induced rat arthritis model. International Journal of Molecular Sciences, 19(1), 203. https://doi.org/10.3390/ijms19010203.
Meretoja, V. V., et al. (2014). Articular chondrocyte redifferentiation in 3D co-cultures with mesenchymal stem cells. Tissue Engineering Part C: Methods, 20(6), 514–523. https://doi.org/10.1089/ten.tec.2013.0532.
Cooke, M. E., et al. (2011). Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis and Cartilage, 19(10), 1210–1218. https://doi.org/10.1016/j.joca.2011.07.005.
Mo, X., et al. (2009). Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone, 45(1), 42–51. https://doi.org/10.1016/j.bone.2008.07.240.
Cho, H., Kim, D., & Kim, K. (2018). Engineered co-culture strategies using stem cells for facilitated chondrogenic differentiation and cartilage repair. Biotechnology and Bioprocess Engineering, 23(3), 261–270. https://doi.org/10.1007/s12257-018-0149-0.
Fernandes, A. M., et al. (2013). Similar properties of chondrocytes from osteoarthritis joints and mesenchymal stem cells from healthy donors for tissue engineering of articular cartilage. PLoS One, 8(5), e62994. https://doi.org/10.1371/journal.pone.0062994.
Vonk, L. A., et al. (2018, 2018). Mesenchymal Stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics, 8(4), 906–920. https://doi.org/10.7150/thno.20746.
Levorson, E. J., et al. (2014). Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomaterialia, 10(5), 1824–1835. https://doi.org/10.1016/j.actbio.2013.12.026.
Tsuchiya, K., et al. (2004). The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Materials Science and Engineering: C, 24(3), 391–396. https://doi.org/10.1016/j.msec.2003.12.014.
Yoshioka, T., et al. (2013). Long-term results of cartilage repair after allogeneic transplantation of cartilaginous aggregates formed from bone marrow–derived cells for large osteochondral defects in rabbit knees. Cartilage, 4(4), 339–344. https://doi.org/10.1177/1947603513494003.
Martin, I., et al. (2001). Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis and Cartilage, 9(2), 112–118. https://doi.org/10.1053/joca.2000.0366.
Acharya, C., et al. (2012). Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. Journal of Cellular Physiology, 227(1), 88–97. https://doi.org/10.1002/jcp.22706.
Chen, Y.-C., et al. (2018). Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PLoS One, 13(11), e0205563. https://doi.org/10.1371/journal.pone.0205563.
Scalzone, A., et al. (2019). The interplay between chondrocyte spheroids and mesenchymal stem cells boosts cartilage regeneration within a 3D natural-based hydrogel. Scientific Reports, 9(1), 14630. https://doi.org/10.1038/s41598-019-51070-7.
Hubka, K. M., et al. (2014). 2014/12//). Enhancing Chondrogenic Phenotype for Cartilage Tissue Engineering: Monoculture and Coculture of Articular Chondrocytes and Mesenchymal Stem Cells. Tissue Engineering Part B: Reviews, 20(6), 641–654. https://doi.org/10.1089/ten.teb.2014.0034.
Wu, L., et al. (2011). Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Engineering Part A, 17(9–10), 1425–1436. https://doi.org/10.1089/ten.tea.2010.0517.
Vonk, L. A., et al. (2010). Preservation of the chondrocyte’s pericellular matrix improves cell-induced cartilage formation. Journal of Cellular Biochemistry. https://doi.org/10.1002/jcb.22533.
Bekkers, J. E. J., et al. (2013). Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: Comparison with microfracture. The American Journal of Sports Medicine, 41(9), 2158–2166. https://doi.org/10.1177/0363546513494181.
Owida, H. A., et al. (2017). Co-culture of chondrons and mesenchymal stromal cells reduces the loss of collagen VI and improves extracellular matrix production. Histochemistry and Cell Biology, 148(6), 625–638. https://doi.org/10.1007/s00418-017-1602-4.
Nagao, M., et al. (2017). Vascular endothelial growth factor in cartilage development and osteoarthritis. Scientific Reports, 7(1), 13027. https://doi.org/10.1038/s41598-017-13417-w.
Honorati, M. C., Cattini, L., & Facchini, A. (2004). IL-17, IL-1β and TNF-α stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthritis and Cartilage, 12(9), 683–691. https://doi.org/10.1016/j.joca.2004.05.009.
Goessler, U. R., et al. (2005). In vitro analysis of matrix proteins and growth factors in dedifferentiating human chondrocytes for tissue-engineered cartilage. Acta Oto-Laryngologica, 125(6), 647–653. https://doi.org/10.1080/00016480510029365.
Mancuso, P., et al. (2019). Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Frontiers in Bioengineering and Biotechnology, 7, 9. https://doi.org/10.3389/fbioe.2019.00009.
Della Bella, E., et al. (2020). Differential regulation of circRNA, miRNA, and piRNA during early osteogenic and chondrogenic differentiation of human mesenchymal stromal cells. Cells, 9(2), 398. https://doi.org/10.3390/cells9020398.
Browe, D. C., et al. (2019). Hypoxia activates the PTHrP –MEF2C pathway to attenuate hypertrophy in mesenchymal stem cell derived cartilage. Scientific Reports, 9(1), 13274. https://doi.org/10.1038/s41598-019-49499-x.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name!: mesenchymal stem cells. Stem Cells Translational Medicine, 6(6), 1445–1451. https://doi.org/10.1002/sctm.17-0051.
Kehl, D., et al. (2019). Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential. NPJ Regenerative Medicine, 4(1), 8. https://doi.org/10.1038/s41536-019-0070-y.
Strioga, M., et al. (2012). Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells and Development, 21(14), 2724–2752. https://doi.org/10.1089/scd.2011.0722.
Zuo, Q., et al. (2013). Co-cultivated mesenchymal stem cells support chondrocytic differentiation of articular chondrocytes. International Orthopaedics, 37(4), 747–752. https://doi.org/10.1007/s00264-013-1782-z.
de Windt, T. S., et al. (2015). Direct cell–cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Engineering Part A, 21(19–20), 2536–2547. https://doi.org/10.1089/ten.tea.2014.0673.
Diao, H. J., et al. (2013). Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regenerative Medicine, 8(3), 257–269. https://doi.org/10.2217/rme.13.22.
Bian, L., et al. (2011). Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Engineering Part A, 17(7–8), 1137–1145. https://doi.org/10.1089/ten.tea.2010.0531.
Liu, X., et al. (2010). In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials, 31(36), 9406–9414. https://doi.org/10.1016/j.biomaterials.2010.08.052.
Zhang, Z., et al. (2018). Mesenchymal stem cells induced by microencapsulated chondrocytes on repairing of intervertebral disc degeneration. Orthopaedic Surgery, 10(4), 328–336. https://doi.org/10.1111/os.12411.
Giovannini, S., et al. (2010). Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. European Cells and Materials, 20, 245–259. https://doi.org/10.22203/eCM.v020a20.
Dahlin, R. L., et al. (2014). 2014/08//). Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials, 35(26), 7460–7469. https://doi.org/10.1016/j.biomaterials.2014.05.055.
Buda, R., et al. (2010). Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. The Journal of Bone and Joint Surgery-American Volume, 92(Suppl 2), 2–11. https://doi.org/10.2106/JBJS.J.00813.
Gobbi, A., et al. (2011). One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: Results at 2-year follow-up. Cartilage, 2(3), 286–299. https://doi.org/10.1177/1947603510392023.
Gobbi, A., Karnatzikos, G., & Sankineani, S. R. (2014). One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. The American Journal of Sports Medicine, 42(3), 648–657. https://doi.org/10.1177/0363546513518007.
Park, Y.-B., et al. (2017). Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up: MSCs for cartilage regeneration in osteoarthritis. Stem Cells Translational Medicine, 6(2), 613–621. https://doi.org/10.5966/sctm.2016-0157.
de Windt, T. S., et al. (2017). Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: A first-in-man trial in 35 patients: Allogeneic MSCs augment one-stage cartilage repair. Stem Cells, 35(8), 1984–1993. https://doi.org/10.1002/stem.2657.
Saris, T. F. F., et al. (2021). Five-year outcome of 1-stage cell-based cartilage repair using recycled autologous chondrons and allogenic mesenchymal stromal cells: A first-in-human clinical trial. The American Journal of Sports Medicine, 49(4), 941–947. https://doi.org/10.1177/0363546520988069.
Korpershoek, J. V., et al. (2020). Efficacy of one-stage cartilage repair using allogeneic mesenchymal stromal cells and autologous chondron transplantation (IMPACT) compared to nonsurgical treatment for focal articular cartilage lesions of the knee: study protocol for a crossover randomized controlled trial. Trials, 21(1), 842–842. https://doi.org/10.1186/s13063-020-04771-8.
Comparing clinical outcomes of the one-step cartilage transplantation in cartilage defects of the knee with conservative treatment. Available from: https://ClinicalTrials.gov/show/NCT04236739.
Leyh, M., et al. (2014). Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Research & Therapy, 16(5), 453. https://doi.org/10.1186/s13075-014-0453-9.
Levato, R., et al. (2017). The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomaterialia, 61, 41–53. https://doi.org/10.1016/j.actbio.2017.08.005.
Kubosch, E. J., et al. (2016). The trans-well coculture of human synovial mesenchymal stem cells with chondrocytes leads to self-organization, chondrogenic differentiation, and secretion of TGFβ. Stem Cell Research & Therapy, 7(1), 64. https://doi.org/10.1186/s13287-016-0322-3.
Wu, L., et al. (2012). Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Engineering Part A, 18(15–16), 1542–1551. https://doi.org/10.1089/ten.tea.2011.0715.
Chahla, J., et al. (2016). Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: A systematic review of the literature and study quality analysis. The Journal of Bone and Joint Surgery, 98(18), 1511–1521. https://doi.org/10.2106/JBJS.15.01495.
Mocchi, M., et al. (2020). Veterinary regenerative medicine for musculoskeletal disorders: Can mesenchymal stem/stromal cells and their secretome be the new frontier? Cells, 9(6). https://doi.org/10.3390/cells9061453.
Wang, Y., et al. (2017). Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Research & Therapy, 8(1), 189. https://doi.org/10.1186/s13287-017-0632-0.
Cosenza, S., et al. (2017). Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Scientific Reports, 7(1), 16214. https://doi.org/10.1038/s41598-017-15376-8.
Zhang, S., et al. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis and Cartilage, 24(12), 2135–2140. https://doi.org/10.1016/j.joca.2016.06.022.
Toh, W. S., et al. (2018). MSC exosome works through a protein-based mechanism of action. Biochemical Society Transactions, 46(4), 843–853. https://doi.org/10.1042/BST20180079.
Phinney, D. G., & Pittenger, M. F. (2017). Concise review: MSC-derived exosomes for cell-free therapy: MSC-derived exosomes. Stem Cells, 35(4), 851–858. https://doi.org/10.1002/stem.2575.
Jeon, O. H., et al. (2017). Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine, 23(6), 775–781. https://doi.org/10.1038/nm.4324.
Acknowledgements
The work of T.Z.B. is supported by the Department of Orthopedics and Trauma Surgery, Albert Ludwigs University Medical Center, and the AO Trauma Deutschland Foundation. The work of M.J.S. and A.R.A. is supported by the AO Foundation. M.J.S. is also supported by the Swiss National Science Foundation (Grant number: 31003A_179438).
Author information
Authors and Affiliations
Contributions
Conceptualization A.R.A; Writing – Original Draft T.Z.B., A.R.A.; Writing – Review and Editing E.J.K., H.S., M.J.S., and A.R.A.; Supervision M.J.S. and A.R.A.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brose, T.Z., Kubosch, E.J., Schmal, H. et al. Crosstalk Between Mesenchymal Stromal Cells and Chondrocytes: The Hidden Therapeutic Potential for Cartilage Regeneration. Stem Cell Rev and Rep 17, 1647–1665 (2021). https://doi.org/10.1007/s12015-021-10170-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10170-6