Abstract
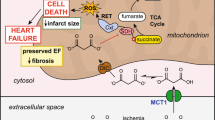
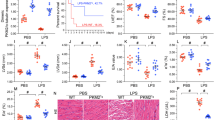
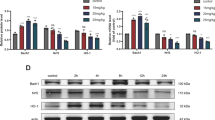
Hydrogen sulfide (H2S) is reported to be effective in the management of the myocardial ischemia–reperfusion (I/R) injury via PI3K/GSK3β pathway in normal rats. However, its efficacy against I/R in the presence of diabetic cardiomyopathy is relatively obscure. Thus, the present work aimed to find out H2S-mediated cardioprotection against I/R in diabetic cardiomyopathy and to evaluate its mode of action using Langendorff isolated heart perfusion system. The present work includes three groups of rat, viz. (i) normal, (ii) diabetes mellitus (DM: streptozotocin: 35 mg/kg; normal diet), and (iii) diabetes + high-fat diet (DCM) (streptozotocin: 35 mg/kg; high-fat diet). The effect of NaHS (an H2S donor; 20 µM) on cardiac function in isolated rat hearts demonstrates that H2S preconditioning (HIPC) significantly attenuated myocardial injury in both DM and DCM hearts, as evidenced by the (i) improvement in hemodynamics, which includes rate pressure product [(in mmHg × 103 × bpm) DM: 40 to 56; DCM: 21 to 58] and left ventricular developed pressure [(in mmHg) DM: 53 to 74; DCM: 28 to 74), (ii) reduction in infarct size (25% to 8%) and attenuated caspase activity, compared to their respective I/R controls. Also, the observed positive recovery of mitochondrial function during HIPC treatment reinforces the cardioprotection by HIPC in DCM heart against I/R injury. However, HIPC could not repair I/R-induced oxidative stress in DCM rat heart. Further, to study the H2S mode of action, the experimental rats were exposed to a PI3K inhibitor (Wortmannin) and GSK3β inhibitor (SB216763) before HIPC protocol, whose results suggest that unlike in normal and DM, HIPC mediates its cardioprotective effect independent of PI3K/GSK3β pathway. To conclude, HIPC ameliorates I/R injury in DCM rat via an alternative pathway other than existing PI3K pathway, which is required to be probed under disease conditions.






Similar content being viewed by others
References
Hausenloy, D. J., & Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. Journal of Clinical Investigation,123(1), 92.
Heusch, G., Boengler, K., & Schulz, R. (2010). Inhibition of mitochondrial permeability transition pore opening: The Holy Grail of cardioprotection. Basic Research in Cardiology,105(2), 151–154.
Grundy, S. M., Benjamin, I. J., Burke, G. L., Chait, A., Eckel, R. H., Howard, B. V., et al. (1999). Diabetes and cardiovascular disease: A statement for health professionals from the American Heart Association. Circulation,100(10), 1134–1146.
Vinik, A. I., Erbas, T., & Casellini, C. M. (2013). Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. Journal of Diabetes Investigation,4(1), 4–18.
Rubler, S., Dlugash, J., Yuceoglu, Y. Z., Kumral, T., Branwood, A. W., & Grishman, A. (1972). New type of cardiomyopathy associated with diabetic glomerulosclerosis. American Journal of Cardiology,30(6), 595–602.
Kannel, W. B., & McGee, D. L. (1979). Diabetes and cardiovascular risk factors: The Framingham study. Circulation,59(1), 8–13.
Andreadou, I., Iliodromitis, E. K., Rassaf, T., Schulz, R., Papapetropoulos, A., & Ferdinandy, P. (2015). The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. British Journal of Pharmacology,172(6), 1587–1606.
Lambert, J. P., Nicholson, C. K., Amin, H., Amin, S., & Calvert, J. W. (2014). Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med. Gas Res.,4, 20.
Peake, B. F., Nicholson, C. K., Lambert, J. P., Hood, R. L., Amin, H., Amin, S., et al. (2013). Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. American Journal of Physiology-Heart and Circulatory Physiology,304(9), H1215–H1224.
Guo, R., Wu, Z., Jiang, J., Liu, C., Wu, B., Li, X., et al. (2017). New mechanism of lipotoxicity in diabetic cardiomyopathy: Deficiency of Endogenous H2S Production and ER stress. Mechanisms of Ageing and Development,162, 46–52.
Zhou, X., An, G., & Lu, X. (2015). Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clinical Science,128(5), 325–335.
Mensah, K., Mocanu, M. M., & Yellon, D. M. (2005). Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: A potential role for phosphatase and tensin homolog deleted on chromosome ten. Journal of the American College of Cardiology,45(8), 1287–1291.
Kurian, G. A., Sachu, P., & Thomas, V. (2005). Effect of aqueous extract of Desmodium Gangeticum root in the severity of isoproterenol induced Myocardial infarcted rats. Journal of Ethnopharmacology,97(3), 457–461.
Noltmann, E. A., Gubler, C. J., & Kuby, S. A. (1961). Glucose 6-phosphate dehydrogenase (Zwischenferment) I. Isolation of the crystalline enzyme from yeast. Journal of Biologiocal Chemistry,236(5), 1225–1230.
Bergmeyer, H. U., Grassl, M., & Walter, H. E. (1983). Reagents for enzymatic analysis. Methods of Enzymatic Analysis,2, 185–223.
Gostimskaya, I., & Galkin, A. (2010). Preparation of highly coupled rat heart mitochondria. Journal of Visualized Experiments,43, 2202.
Frazier, A. E., & Thorburn, D. R. (2012). Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods in Molecular Biology,837, 49–62.
Fraga, C. G., Leibovitz, B. E., & Tappel, A. L. (1988). Lipid peroxidation measured as thiobarbituric acid reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radical Biology and Medicine,4, 155–161.
Ansari, S. B., & Kurian, G. A. (2016). Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chemico-Biological Interactions,252, 28–35.
Denton, D., Mills, K., & Kumar, S. (2008). Methods and protocols for studying cell death in Drosophila. Methods in Enzymology,446, 17–37.
Stepien, P. P., & Pieniazek, N. J. (1973). The use of the l-serine sulfhydrylase assay for the estimation of cystathionine b-synthase. Analytical Biochemistry,54, 294–299.
Lee, C. H., Hwang, J. H., Lee, Y. S., & Cho, K. S. (1995). Purification and characterization of mouse liver rhodanase. Journal of Biochemistry and Molecular Biology,28, 170–176.
Elrod, J. W., Calvert, J. W., Morrison, J., Doeller, J. E., Kraus, D. W., Tao, L., et al. (2007). Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America,104(39), 5560–15565.
Karwi, Q. G., Bornbaum, J., Boengler, K., Torregrossa, R., Whiteman, M., Wood, M. E., et al. (2017). AP39, a mitochondria-targeting hydrogen sulfide (H2S) donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. British Journal of Pharmacology,174, 287–301.
Chatzianastasiou, A., Bibli, S. I., Andreadou, I., Efentakis, P., Kaludercic, N., Wood, M. E., et al. (2016). Cardioprotection by H2S donors: nitric oxide-dependent and -independent mechanisms. Journal of Pharmacology and Experimental Therapeutics,106, 432–442.
Sivarajah, A., Collino, M., Yasin, M., Benetti, E., Gallicchio, M., Mazzon, E., et al. (2009). Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock,31, 267–274.
Yao, L. L., Huang, X. W., Wang, Y. G., Cao, Y. X., Zhang, C. C., & Zhu, Y. C. (2010). Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of mPTP. American Journal of Physiology-Heart and Circulatory Physiology,298, H1310–H1319.
Buchanan, J., Mazumder, P. K., Hu, P., Chakrabarti, G., Roberts, M. W., Yun, U. J., et al. (2005). Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology,146, 5341–5349.
Ouwens, D. M., Boer, C., Fodor, M., De Galan, P., Heine, R. J., Maassen, J. A., et al. (2005). Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia,48, 1229–1237.
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E., & Blaxall, B. C. (2016). Cardiac fibrosis: the fibroblast awakens. Circulation Research,118(6), 1021–1040.
Frangogiannis, N. G. (2012). Regulation of the inflammatory response in cardiac repair. Circulation Research,110, 159–173.
Yang, Z., Tian, Y., Liu, Y., Hennessy, S., Kron, I. L., & French, B. A. (2013). Acute hyperglycemia abolishes ischemic preconditioning by inhibiting Akt phosphorylation: Normalizing blood glucose before ischemia restores ischemic preconditioning. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2013/329183.
Jiang, H., Xiao, J., Kang, B., Zhu, X., Xin, N., & Wang, Z. (2016). PI3K/SGK1/GSK3β signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Experimental Cell Research,345(2), 134–140.
Heydrick, S. J., Jullien, D., Gautier, N., Tanti, J. F., Giorgetti, S., Van Obberghen, E., et al. (1993). Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. Journal of Clinical Investigation,91(4), 1358.
Boudina, S., Sena, S., O’Neill, B. T., Tathireddy, P., Young, M. E., & Abel, E. D. (2005). Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation,112, 2686–2695.
Bugger, H., Boudina, S., Hu, X. X., Tuinei, J., Zaha, V. G., Theobald, H. A., et al. (2008). Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes,57, 2924–2932.
Duncan, J. G., Fong, J. L., Medeiros, D. M., Finck, B. N., & Kelly, D. P. (2007). Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation,115, 909–917.
Ansari, M., Gopalakrishnan, S., & Kurian, G. A. (2019). Streptozotocin-induced type II diabetic rat administered with nonobesogenic high-fat diet is highly susceptible to myocardial ischemia–reperfusion injury: An insight into the function of mitochondria. Journal of Cellular Physiology,234(4), 4104–4114.
Tian, D., Dong, J., Jin, S., Teng, X., & Wu, Y. (2017). Endogenous hydrogen sulfide-mediated MAPK inhibition preserves endothelial function through TXNIP signaling. Free Radical Biology and Medicine,110, 291–299.
Xie, Z. Z., Liu, Y., & Bian, J. S. (2016). Hydrogen sulfide and cellular redox homeostasis. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2016/6043038.
Mahalakshmi, A., & Kurian, G. A. (2018). Evaluating the impact of diabetes and diabetic cardiomyopathy rat heart on the outcome of ischemia-reperfusion associated oxidative stress. Free Radical Biology and Medicine,118, 35–43.
Szabo, C., Ransy, C., Módis, K., Andriamihaja, M., Murghes, B., Coletta, C., et al. (2014). Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British Journal of Pharmacology,171, 2099–2122.
Fu, M., Zhang, W., Wu, L., Yang, G., Li, H., & Wang, R. (2012). Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proceedings of the National Academy of Sciences United States of America,109(8), 2943–2948.
Bravo, E., Palleschi, S., Aspichueta, P., Buqué, X., Rossi, B., Cano, A., et al. (2011). High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids in Health and Disease,10(1), 60.
Acknowledgements
The authors sincerely thank the Department of Science and Technology (DST/INSPIRE Fellowship/2013/326). The authors also place on record their thanks to Kausthubh R. for his editing of this manuscript.
Author information
Authors and Affiliations
Contributions
Dr. Gino A Kurian has contributed to the design and implementation of the research, the interpretation of the results, and writing of the manuscript. Ms. Mahalakshmi has processed the experimental data, performed analysis, drafted the manuscript, designed figures and tables, and compiled the literature sources.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Handling Editor: Filomain Nguemo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ansari, M., Kurian, G.A. Mechanism of Hydrogen Sulfide Preconditioning-Associated Protection Against Ischemia–Reperfusion Injury Differs in Diabetic Heart That Develops Myopathy. Cardiovasc Toxicol 20, 155–167 (2020). https://doi.org/10.1007/s12012-019-09542-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-019-09542-9